Oxidative stress mediates the disruption of airway epithelial tight junctions through a TRPM2-PLCγ1-PKCα signaling pathway
- PMID: 23629676
- PMCID: PMC3676794
- DOI: 10.3390/ijms14059475
Oxidative stress mediates the disruption of airway epithelial tight junctions through a TRPM2-PLCγ1-PKCα signaling pathway
Abstract
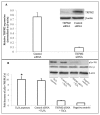
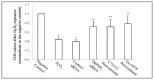
Oxidative stress has been implicated as an important contributing factor in the pathogenesis of several pulmonary inflammatory diseases. Previous studies have indicated a relationship between oxidative stress and the attenuation of epithelial tight junctions (TJs). In Human Bronchial Epithelial-16 cells (16HBE), we demonstrated the degradation of zonula occludens-1 (ZO-1), and claudin-2 exhibited a great dependence on the activation of the transient receptor potential melastatin (TRPM) 2 channel, phospholipase Cγ1 (PLCγ1) and the protein kinase Cα (PKCα) signaling cascade.
Figures


Similar articles
-
Alcohol increases the permeability of airway epithelial tight junctions in Beas-2B and NHBE cells.Alcohol Clin Exp Res. 2012 Mar;36(3):432-42. doi: 10.1111/j.1530-0277.2011.01640.x. Epub 2011 Sep 26. Alcohol Clin Exp Res. 2012. PMID: 21950588 Free PMC article.
-
The degradation of airway tight junction protein under acidic conditions is probably mediated by transient receptor potential vanilloid 1 receptor.Biosci Rep. 2013 Oct 31;33(5):e00078. doi: 10.1042/BSR20130087. Biosci Rep. 2013. PMID: 24073800 Free PMC article.
-
Cigarette smoke-induced disruption of bronchial epithelial tight junctions is prevented by transforming growth factor-β.Am J Respir Cell Mol Biol. 2014 Jun;50(6):1040-52. doi: 10.1165/rcmb.2013-0090OC. Am J Respir Cell Mol Biol. 2014. PMID: 24358952
-
TRPM2 channel regulates endothelial barrier function.Adv Exp Med Biol. 2010;661:155-67. doi: 10.1007/978-1-60761-500-2_10. Adv Exp Med Biol. 2010. PMID: 20204729 Review.
-
The Epithelial Cell Leak Pathway.Int J Mol Sci. 2021 Jul 18;22(14):7677. doi: 10.3390/ijms22147677. Int J Mol Sci. 2021. PMID: 34299297 Free PMC article. Review.
Cited by
-
Dust particles-induced intracellular Ca2+ signaling and reactive oxygen species in lung fibroblast cell line MRC5.Korean J Physiol Pharmacol. 2017 May;21(3):327-334. doi: 10.4196/kjpp.2017.21.3.327. Epub 2017 Apr 21. Korean J Physiol Pharmacol. 2017. PMID: 28461775 Free PMC article.
-
Exposure to inhomogeneous static magnetic field beneficially affects allergic inflammation in a murine model.J R Soc Interface. 2014 Mar 19;11(95):20140097. doi: 10.1098/rsif.2014.0097. Print 2014 Jun 6. J R Soc Interface. 2014. PMID: 24647908 Free PMC article. Clinical Trial.
-
The role of stretch-activated ion channels in acute respiratory distress syndrome: finally a new target?Am J Physiol Lung Cell Mol Physiol. 2016 Sep 1;311(3):L639-52. doi: 10.1152/ajplung.00458.2015. Epub 2016 Aug 12. Am J Physiol Lung Cell Mol Physiol. 2016. PMID: 27521425 Free PMC article. Review.
-
Probiotic Properties and Safety Evaluation of Lactobacillus plantarum HY7718 with Superior Storage Stability Isolated from Fermented Squid.Microorganisms. 2023 Sep 8;11(9):2254. doi: 10.3390/microorganisms11092254. Microorganisms. 2023. PMID: 37764098 Free PMC article.
-
The Effect of Therapeutic Blockades of Dust Particles-Induced Ca²⁺ Signaling and Proinflammatory Cytokine IL-8 in Human Bronchial Epithelial Cells.Mediators Inflamm. 2015;2015:843024. doi: 10.1155/2015/843024. Epub 2015 Nov 10. Mediators Inflamm. 2015. PMID: 26640326 Free PMC article.
References
-
- Tomita K., Barnes P.J., Adcock I.M. The effect of oxidative stress on histone acetylation and IL-8 release. Biochem. Biophys. Res. Commun. 2003;301:572–577. - PubMed
-
- Van Itallie C.M., Anderson J.M. The molecular physiology of tight junction pores. Physiology. 2004;19:331–338. - PubMed
-
- Balda M.S., Matter K. Tight junctions and the regulation of gene expression. Biochim. Biophys. Acta. 2009;1788:761–767. - PubMed
-
- Wang F., Daugherty B., Keise L.L., Wei Z., Foley J.P., Savani R.C., Koval M. Heterogeneity of claudin expression by alveolar epithelial cells. Am. J. Respir. Cell Mol. Biol. 2003;29:62–70. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

