Pharmacokinetics-pharmacodynamics of tazobactam in combination with ceftolozane in an in vitro infection model
- PMID: 23629705
- PMCID: PMC3716129
- DOI: 10.1128/AAC.02513-12
Pharmacokinetics-pharmacodynamics of tazobactam in combination with ceftolozane in an in vitro infection model
Abstract
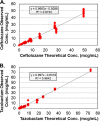
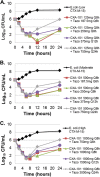
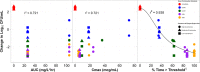
Despite β-lactamase inhibitors being available for clinical use for nearly 30 years, a paucity of data exists describing the pharmacokinetic-pharmacodynamic (PK-PD) determinants of efficacy for these agents. Herein, we describe dose fractionation studies designed to determine the exposure measure most predictive of tazobactam efficacy in combination with ceftolozane and the magnitude of this measure necessary for efficacy in a PK-PD in vitro infection model. The challenge organism panel was comprised of an isogenic CTX-M-15-producing Escherichia coli triplet set, genetically engineered to transcribe different levels of bla(CTX-M-15). These recombinant strains exhibited ceftolozane MIC values of 4, 16, and 64 μg/ml representing low, moderate, and high levels of CTX-M-15, respectively. Different bla(CTX-M-15) transcription levels were confirmed by relative quantitative real-time PCR (qRT-PCR) and β-lactamase hydrolytic assays. The exposure measure associated with efficacy was the percentage of the dosing interval that tazobactam concentrations remained above a threshold (%Time>threshold), regardless of enzyme expression (r(2) = 0.938). The threshold concentrations identified were 0.05 μg/ml for low and moderate and 0.25 μg/ml for the high-β-lactamase expression strain constructs. The magnitudes of %Time>threshold for tazobactam associated with net bacterial stasis and a 1- and 2-log10 CFU reduction in bacteria at 24 h were approximately 35, 50, and 70%, respectively. These data provide an initial target tazobactam concentration-time profile and a paradigm to optimize tazobactam dosing when combined with ceftolozane.
Figures

References
-
- Todd PA, Benfield P.1990. Amoxicillin/clavulanic acid. An update of its antibacterial activity, pharmacokinetic properties and therapeutic use. Drugs 39:264–307 - PubMed
-
- Davies BI, Maesen FP, van Noord JA. 1984. Clinical, bacteriological and pharmacokinetic results from an open trial of sultamicillin in patients with acute exacerbations of chronic bronchitis. J. Antimicrob. Chemother. 13:161–170 - PubMed
-
- Jackson D, Cockburn A, Cooper DL, Langley PF, Tasker TC, White DJ. 1985. Clinical pharmacology and safety evaluation on timentin. Am. J. Med. 79:44–55 - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
- Actions
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

