Caveolae-mediated endocytosis of conjugated polymer nanoparticles
- PMID: 23629923
- PMCID: PMC3787714
- DOI: 10.1002/mabi.201300030
Caveolae-mediated endocytosis of conjugated polymer nanoparticles
Abstract
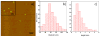
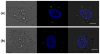
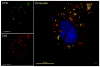
Understanding the cellular entry pathways of synthetic biomaterials is highly important to improve overall labeling and delivery efficiency. Herein, cellular entry mechanisms of conjugated polymer nanoparticles (CPNs) are presented. CPNs are intrinsic fluorescent materials used for various biological applications. While CPNs cause no toxicity, decreased CPN uptake is observed from cancer cells pretreated with genistein, which is an inhibitor of caveolae-mediated endocytosis (CvME). CvME is further confirmed by high co-localization with caveolin-1 proteins found in the caveolae and caveosomes. Excellent photophysical properties, non-toxicity, and non-destructive delivery pathways support that CPNs are promising multifunctional carriers minimizing degradation of contents during delivery.
Keywords: conjugated polyelectrolytes; conjugated polymer nanoparticles; conjugated polymers; endocytosis mechanism; small interfering RNA delivery.
© 2013 WILEY-VCH Verlag GmbH & Co. KGaA, Weinheim.
Figures
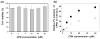
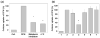


References
-
- Napp J, Behnke T, Fischer L, Wurth C, Wottawa M, Katschinski DM, Alves F, Resch-Genger U, Schaferling M. Anal Chem. 2011;83:9039–9046. - PubMed
- Ye FM, Wu CF, Jin YH, Chan YH, Zhang XJ, Chiu DT. J Am Chem Soc. 2011;133:8146–8149. - PMC - PubMed
- Kim JH, Lee S, Park K, Nam HY, Jang SY, Youn I, Kim K, Jeon H, Park RW, Kim IS, Choi K, Kwon IC. Angew Chem Int Ed. 2007;46:5779–5782. - PubMed
-
- Lynn DM, Langer R. J Am Chem Soc. 2000;122:10761–10768.
- Choi JS, Nam K, Park J, Kim JB, Lee JK, Park J. J Control Release. 2004;99:445–456. - PubMed
- Pun SH, Bellocq NC, Liu AJ, Jensen G, Machemer T, Quijano E, Schluep T, Wen SF, Engler H, Heidel J, Davis ME. Bioconjugate Chem. 2004;15:831–840. - PubMed
- Srinivasachari S, Fichter KM, Reineke TM. J Am Chem Soc. 2008;130:4618–4627. - PubMed
- Ornelas-Megiatto C, Wich PR, Frechet JMJ. J Am Chem Soc. 2012;134:1902–1905. - PubMed
- Silva AT, Alien N, Ye CM, Verchot J, Moon JH. BMC PlantBiol. 2010;10:291. - PMC - PubMed
- Moon JH, Mendez E, Kim Y, Kaur A. Chem Comm. 2011;47:8370–8372. - PubMed
-
- Nel AE, Madler L, Velegol D, Xia T, Hoek EMV, Somasundaran P, Klaessig F, Castranova V, Thompson M. Nat Mater. 2009;8:543–557. - PubMed
-
- Tan SJ, Jana NR, Gao SJ, Patra PK, Ying JY. Chem Mater. 2010;22:2239–2247.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

