Differential hERG ion channel activity of ultrasmall gold nanoparticles
- PMID: 23630249
- PMCID: PMC3657833
- DOI: 10.1073/pnas.1220143110
Differential hERG ion channel activity of ultrasmall gold nanoparticles
Abstract
Understanding the mechanism of toxicity of nanomaterials remains a challenge with respect to both mechanisms involved and product regulation. Here we show toxicity of ultrasmall gold nanoparticles (AuNPs). Depending on the ligand chemistry, 1.4-nm-diameter AuNPs failed electrophysiology-based safety testing using human embryonic kidney cell line 293 cells expressing human ether-á-go-go-Related gene (hERG), a Food and Drug Administration-established drug safety test. In patch-clamp experiments, phosphine-stabilized AuNPs irreversibly blocked hERG channels, whereas thiol-stabilized AuNPs of similar size had no effect in vitro, and neither particle blocked the channel in vivo. We conclude that safety regulations may need to be reevaluated and adapted to reflect the fact that the binding modality of surface functional groups becomes a relevant parameter for the design of nanoscale bioactive compounds.
Keywords: complementarity; gold cluster; nanotoxicology; shape.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

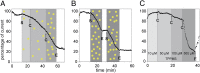
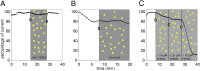


References
-
- Daniel M-C, Astruc D. Gold nanoparticles: Assembly, supramolecular chemistry, quantum-size-related properties, and applications toward biology, catalysis, and nanotechnology. Chem Rev. 2004;104(1):293–346. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

