Gene silencing in adipose tissue macrophages regulates whole-body metabolism in obese mice
- PMID: 23630254
- PMCID: PMC3657808
- DOI: 10.1073/pnas.1300492110
Gene silencing in adipose tissue macrophages regulates whole-body metabolism in obese mice
Abstract
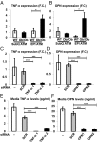
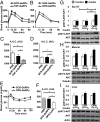
Adipose tissue (AT) inflammation and infiltration by macrophages is associated with insulin resistance and type 2 diabetes in obese humans, offering a potential target for therapeutics. However, whether AT macrophages (ATMs) directly contribute to systemic glucose intolerance has not been determined. The reason is the lack of methods to ablate inflammatory genes expressed in macrophages specifically localized within AT depots, leaving macrophages in other tissues unaffected. Here we report that i.p. administration of siRNA encapsulated by glucan shells in obese mice selectively silences genes in epididymal ATMs, whereas macrophages within lung, spleen, kidney, heart, skeletal muscle, subcutaneous (SubQ) adipose, and liver are not targeted. Such administration of GeRPs to silence the inflammatory cytokines TNF-α or osteopontin in epididymal ATMs of obese mice caused significant improvement in glucose tolerance. These data are consistent with the hypothesis that cytokines produced by ATMs can exacerbate whole-body glucose intolerance.
Keywords: RNAi; immune cells; obesity.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



References
-
- Chen L, Magliano DJ, Zimmet PZ. [The worldwide epidemiology of type 2 diabetes mellitus—present and future perspectives.] Nat Rev Endocrinol. 2011;8(4):228–236. - PubMed
-
- Kusminski CM, Shetty S, Orci L, Unger RH, Scherer PE. Diabetes and apoptosis: Lipotoxicity. Apoptosis. 2009;14(12):1484–1495. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

