Glycolytic strategy as a tradeoff between energy yield and protein cost
- PMID: 23630264
- PMCID: PMC3683749
- DOI: 10.1073/pnas.1215283110
Glycolytic strategy as a tradeoff between energy yield and protein cost
Abstract
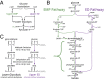
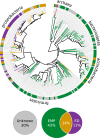
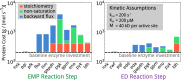
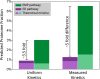
Contrary to the textbook portrayal of glycolysis as a single pathway conserved across all domains of life, not all sugar-consuming organisms use the canonical Embden-Meyerhoff-Parnass (EMP) glycolytic pathway. Prokaryotic glucose metabolism is particularly diverse, including several alternative glycolytic pathways, the most common of which is the Entner-Doudoroff (ED) pathway. The prevalence of the ED pathway is puzzling as it produces only one ATP per glucose--half as much as the EMP pathway. We argue that the diversity of prokaryotic glucose metabolism may reflect a tradeoff between a pathway's energy (ATP) yield and the amount of enzymatic protein required to catalyze pathway flux. We introduce methods for analyzing pathways in terms of thermodynamics and kinetics and show that the ED pathway is expected to require several-fold less enzymatic protein to achieve the same glucose conversion rate as the EMP pathway. Through genomic analysis, we further show that prokaryotes use different glycolytic pathways depending on their energy supply. Specifically, energy-deprived anaerobes overwhelmingly rely upon the higher ATP yield of the EMP pathway, whereas the ED pathway is common among facultative anaerobes and even more common among aerobes. In addition to demonstrating how protein costs can explain the use of alternative metabolic strategies, this study illustrates a direct connection between an organism's environment and the thermodynamic and biochemical properties of the metabolic pathways it employs.
Keywords: enzyme cost; evolution.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


Comment in
-
The cost of efficiency in energy metabolism.Proc Natl Acad Sci U S A. 2013 Jun 11;110(24):9629-30. doi: 10.1073/pnas.1307485110. Epub 2013 May 31. Proc Natl Acad Sci U S A. 2013. PMID: 23729810 Free PMC article. No abstract available.
References
-
- Romano AH, Conway T. Evolution of carbohydrate metabolic pathways. Res Microbiol. 1996;147(6-7):448–455. - PubMed
-
- Kim BH, Gadd GM. Bacterial Physiology and Metabolism. Cambridge, UK: Cambridge Univ Press; 2008.
-
- Fraenkel D. Escherichia coli and Salmonella. In: Neidhardt FC, editor. Cellular and Molecular Biology. Vol. 1. Washington, DC: American Society for Microbiology; 1996. pp. 189–199.
-
- Conway T. The Entner-Doudoroff pathway: History, physiology and molecular biology. FEMS Microbiol Rev. 1992;9(1):1–27. - PubMed
-
- Meléndez-Hevia E, Waddell TG, Heinrich R, Montero F. Theoretical approaches to the evolutionary optimization of glycolysis—chemical analysis. Eur J Biochem. 1997;244(2):527–543. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

