Mechanism of somatic hypermutation at the WA motif by human DNA polymerase η
- PMID: 23630267
- PMCID: PMC3657764
- DOI: 10.1073/pnas.1303126110
Mechanism of somatic hypermutation at the WA motif by human DNA polymerase η
Abstract
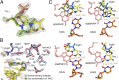
Somatic hypermutation is programmed base substitutions in the variable regions of Ig genes for high-affinity antibody generation. Two motifs, RGYW and WA (R, purine; Y, pyrimidine; W, A or T), have been found to be somatic hypermutation hotspots. Overwhelming evidence suggests that DNA polymerase η (Pol η) is responsible for converting the WA motif to WG by misincorporating dGTP opposite the templating T. To elucidate the molecular mechanism, crystal structures and kinetics of human Pol η substituting dGTP for dATP in four sequence contexts, TA, AA, GA, and CA, have been determined and compared. The T:dGTP wobble base pair is stabilized by Gln-38 and Arg-61, two uniquely conserved residues among Pol η. Weak base paring of the W (T:A or A:T) at the primer end and their distinct interactions with Pol η lead to misincorporation of G in the WA motif. Between two WA motifs, our kinetic and structural data indicate that A-to-G mutation occurs more readily in the TA context than AA. Finally, Pol η can extend the T:G mispair efficiently to complete the mutagenesis.
Keywords: A-to-G transition; immunoglobulin; π–cation stacking.
Conflict of interest statement
The authors declare no conflict of interest.
Figures




References
-
- Rajewsky K, Förster I, Cumano A. Evolutionary and somatic selection of the antibody repertoire in the mouse. Science. 1987;238(4830):1088–1094. - PubMed
-
- Kim S, Davis M, Sinn E, Patten P, Hood L. Antibody diversity: Somatic hypermutation of rearranged VH genes. Cell. 1981;27(3 Pt 2):573–581. - PubMed
-
- Di Noia JM, Neuberger MS. Molecular mechanisms of antibody somatic hypermutation. Annu Rev Biochem. 2007;76:1–22. - PubMed
-
- Muramatsu M, Nagaoka H, Shinkura R, Begum NA, Honjo T. Discovery of activation-induced cytidine deaminase, the engraver of antibody memory. Adv Immunol. 2007;94:1–36. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

