Steroidogenic enzyme Cyp11a1 regulates Type 2 CD8+ T cell skewing in allergic lung disease
- PMID: 23630275
- PMCID: PMC3657775
- DOI: 10.1073/pnas.1216671110
Steroidogenic enzyme Cyp11a1 regulates Type 2 CD8+ T cell skewing in allergic lung disease
Abstract
Allergic asthma is a heterogeneous inflammatory disorder of the airways characterized by chronic airway inflammation and airway hyperresponsiveness. Numbers of CD8(+)IL-13(+) T cells are increased in asthmatics and during the development of experimental asthma in mice. In an atopic environment rich in IL-4, these CD8(+) T cells mediate asthmatic responses, but the mechanisms regulating the conversion of CD8(+) effector T cells from IFN-γ- to pathogenic IL-13-producing effector cells that contribute to an asthma phenotype have not been defined. Here, we show that cholesterol side-chain cleavage P450 enzyme, Cyp11a1, is a key regulator of CD8(+) T-cell conversion. Expression of the gene, protein, and enzymatic activity of Cyp11a1 were markedly increased in CD8(+) T cells differentiated in the presence of IL-2 plus IL-4 compared with cells differentiated in IL-2 alone. Inhibition of Cyp11a1 enzymatic activity with aminoglutethimide or reduction in the expression of Cyp11a1 using short hairpin RNA prevented the IL-4-induced conversion of IFN-γ- to IL-13-producing cells without affecting expression of the lineage-specific transcription factors T-box expressed in T cells (T-bet) or GATA binding protein 3 (GATA3). Adoptive transfer of aminoglutethimide-treated CD8(+) T cells into sensitized and challenged CD8-deficient recipients failed to restore airway hyperresponsiveness and inflammation. We demonstrate that Cyp11a1 controls the phenotypic conversion of CD8(+) T cells from IFN-γ to IL-13 production, linking steroidogenesis in CD8(+) T cells, a nonclassical steroidogenic tissue, to a proallergic differentiation pathway.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


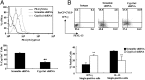
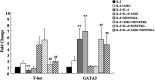

Similar articles
-
Expression and activation of the steroidogenic enzyme CYP11A1 is associated with IL-13 production in T cells from peanut allergic children.PLoS One. 2020 Jun 4;15(6):e0233563. doi: 10.1371/journal.pone.0233563. eCollection 2020. PLoS One. 2020. PMID: 32497050 Free PMC article.
-
1,25D3 prevents CD8(+)Tc2 skewing and asthma development through VDR binding changes to the Cyp11a1 promoter.Nat Commun. 2016 Jan 11;7:10213. doi: 10.1038/ncomms10213. Nat Commun. 2016. PMID: 26750596 Free PMC article.
-
The steroidogenic enzyme Cyp11a1 is essential for development of peanut-induced intestinal anaphylaxis.J Allergy Clin Immunol. 2013 Nov;132(5):1174-1183.e8. doi: 10.1016/j.jaci.2013.05.027. Epub 2013 Jul 16. J Allergy Clin Immunol. 2013. PMID: 23870673 Free PMC article.
-
[Imbalanced T cell-specific transcription factors T-bet and GATA-3 and relationship to airway inflammation in asthmatic rats].Zhonghua Jie He He Hu Xi Za Zhi. 2006 Mar;29(3):176-80. Zhonghua Jie He He Hu Xi Za Zhi. 2006. PMID: 16677481 Chinese.
-
Increased IL-4- and IL-17-producing CD8+ cells are related to decreased CD39+CD4+Foxp3+ cells in allergic asthma.J Asthma. 2018 Jan;55(1):8-14. doi: 10.1080/02770903.2017.1310225. Epub 2017 May 23. J Asthma. 2018. PMID: 28346024
Cited by
-
Insufficient interleukin-12 signalling favours differentiation of human CD4(+) and CD8(+) T cells into GATA-3(+) and GATA-3(+) T-bet(+) subsets in humanized mice.Immunology. 2014 Oct;143(2):202-18. doi: 10.1111/imm.12304. Immunology. 2014. PMID: 24766459 Free PMC article.
-
Expression and activation of the steroidogenic enzyme CYP11A1 is associated with IL-13 production in T cells from peanut allergic children.PLoS One. 2020 Jun 4;15(6):e0233563. doi: 10.1371/journal.pone.0233563. eCollection 2020. PLoS One. 2020. PMID: 32497050 Free PMC article.
-
1,25D3 prevents CD8(+)Tc2 skewing and asthma development through VDR binding changes to the Cyp11a1 promoter.Nat Commun. 2016 Jan 11;7:10213. doi: 10.1038/ncomms10213. Nat Commun. 2016. PMID: 26750596 Free PMC article.
-
T-cell autonomous death induced by regeneration of inert glucocorticoid metabolites.Cell Death Dis. 2017 Jul 20;8(7):e2948. doi: 10.1038/cddis.2017.344. Cell Death Dis. 2017. PMID: 28726773 Free PMC article.
-
CLICK-chemoproteomics and molecular dynamics simulation reveals pregnenolone targets and their binding conformations in Th2 cells.Front Immunol. 2023 Oct 31;14:1229703. doi: 10.3389/fimmu.2023.1229703. eCollection 2023. Front Immunol. 2023. PMID: 38022565 Free PMC article.
References
-
- Holgate ST. Innate and adaptive immune responses in asthma. Nat Med. 2012;18(5):673–683. - PubMed
-
- National Asthma Education and Prevention Program (National Heart Lung and Blood Institute) Third Expert Panel on the Management of Asthma (2007) Expert Panel Report 3: Guidelines for the Diagnosis and Management of Asthma (National Center for Biotechnology Information, US National Library of Medicine, Bethesda), Available at www.ncbi.nlm.nih.gov/books/bv.fcgi?rid=asthma3.TOC&depth=2.
-
- From the Global Strategy for Asthma Management and Prevention, Global Initiative for Asthma (GINA) 2012. Available at http://www.ginasthma.org.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials

