Intermediate intrinsic diversity enhances neural population coding
- PMID: 23630284
- PMCID: PMC3657795
- DOI: 10.1073/pnas.1221214110
Intermediate intrinsic diversity enhances neural population coding
Abstract
Cell-to-cell variability in molecular, genetic, and physiological features is increasingly recognized as a critical feature of complex biological systems, including the brain. Although such variability has potential advantages in robustness and reliability, how and why biological circuits assemble heterogeneous cells into functional groups is poorly understood. Here, we develop analytic approaches toward answering how neuron-level variation in intrinsic biophysical properties of olfactory bulb mitral cells influences population coding of fluctuating stimuli. We capture the intrinsic diversity of recorded populations of neurons through a statistical approach based on generalized linear models. These models are flexible enough to predict the diverse responses of individual neurons yet provide a common reference frame for comparing one neuron to the next. We then use Bayesian stimulus decoding to ask how effectively different populations of mitral cells, varying in their diversity, encode a common stimulus. We show that a key advantage provided by physiological levels of intrinsic diversity is more efficient and more robust encoding of stimuli by the population as a whole. However, we find that the populations that best encode stimulus features are not simply the most heterogeneous, but those that balance diversity with the benefits of neural similarity.
Keywords: generalized linear models; intrinsic biophysics; ion channels; neural variability; stimulus coding.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
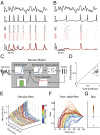
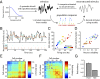
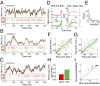

References
-
- Puchalla JL, Schneidman E, Harris RA, Berry MJ. Redundancy in the population code of the retina. Neuron. 2005;46(3):493–504. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

