Regulatory B cells suppress imiquimod-induced, psoriasis-like skin inflammation
- PMID: 23630391
- PMCID: PMC3774845
- DOI: 10.1189/jlb.1112562
Regulatory B cells suppress imiquimod-induced, psoriasis-like skin inflammation
Abstract
Psoriasis is an inflammatory cutaneous disorder characterized by marked epidermal thickening and Th1 and Th17 cell infiltration. At present, the contribution of B cells to the pathogenesis of psoriasis is unclear. In mice, topical application of imiquimod induces inflamed skin lesions and serves as an experimental animal model for human psoriasis. In this study, we showed that imiquimod-induced skin inflammation was more severe in CD19(-/-) than WT mice. These inflammatory responses were negatively regulated by a unique IL-10-producing CD1d(hi)CD5(+) regulatory B cell subset (B10 cells) that was absent in CD19(-/-) mice and represented only 1-2% of splenic B220(+) cells in WT mice. Splenic B10 cells entered the circulation and migrated to draining LNs during imiquimod-induced skin inflammation, thereby suppressing IFN-γ and IL-17 production. Furthermore, adoptive transfer of these B10 cells from WT mice reduced inflammation in CD19(-/-) mice. The present findings provide direct evidence that B10 cells regulate imiquimod-induced skin inflammation and offer insights into regulatory B cell-based therapies for the treatment of psoriasis.
Keywords: B10 cells; IL-10; cytokines.
Figures
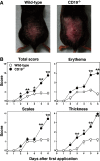
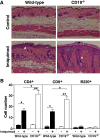
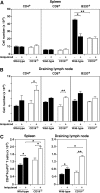

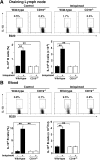

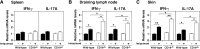

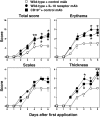
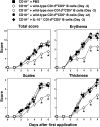
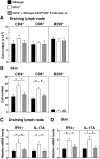
Comment in
-
Editorial: are regulatory B10 cells a viable target for autoimmune diseases?J Leukoc Biol. 2013 Oct;94(4):548-50. doi: 10.1189/jlb.0513267. J Leukoc Biol. 2013. PMID: 24082049 No abstract available.
References
-
- Nestle F. O., Kaplan D. H., Barker J. (2009) Psoriasis. N. Engl. J. Med. 361, 496–09 - PubMed
-
- Lowes M. A., Kikuchi T., Fuentes-Duculan J., Cardinale I., Zaba L. C., Haider A. S., Bowman E. P., Krueger J. G. (2008) Psoriasis vulgaris lesions contain discrete populations of Th1 and Th17 T cells. J. Invest. Dermatol. 128, 1207–1211 - PubMed
-
- Schon M. P., Ruzicka T. (2001) Psoriasis: the plot thickens. Nat. Immunol. 2, 91. - PubMed
-
- Gilliet M., Conrad C., Geiges M., Cozzio A., Thurlimann W., Burg G., Nestle F. O., Dummer R. (2004) Psoriasis triggered by Toll-like receptor 7 agonist imiquimod in the presence of dermal plasmacytoid dendritic cell precursors. Arch. Dermatol. 140, 1490–1495 - PubMed
-
- Patel U., Mark N. M., Machler B. C., Levine V. J. (2011) Imiquimod 5% cream induced psoriasis: a case report, summary of the literature and mechanism. Br. J. Dermatol. 164, 670–672 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials

