Gender difference in response predictors after 1-year exenatide therapy twice daily in type 2 diabetic patients: a real world experience
- PMID: 23630427
- PMCID: PMC3626369
- DOI: 10.2147/DMSO.S42729
Gender difference in response predictors after 1-year exenatide therapy twice daily in type 2 diabetic patients: a real world experience
Abstract
Purpose: To investigate whether gender affects therapeutic response by exenatide twice a day (BID) in type 2 diabetes by using a database concerning patients monitored by five outpatient clinics in Tuscany, Italy.
Patients and methods: We considered a cohort of 315 (154 male/161 female) patients experiencing therapeutic failure while on oral therapy (metformin, or combination therapy metformin + sulphonylureas), who were given exenatide (10 μg/BID) and who fully completed 4 months, 8 months, and 12 months of follow-ups.
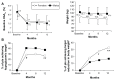
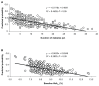
Results: Among patients stratified by gender and well matched for age, body mass index, and hemoglobin A1c (HbA1c), it was found that the length of disease was longer in females than in males (12 ± 8 years versus 10 ± 7 years; P = 0.037), and the ratio of patients on metformin to those on combination therapy was higher in men (P = 0.018). Target glycemic response (1-year HbA1c ≤ 7%) was achieved in a significantly higher proportion of males than females (38% versus 27%; χ(2) = 4.66; P = 0.03). Target weight loss expressed as 1-year weight percent fall from baseline ≥ 75th percentile (8.5%) was significantly higher in females at 8 and 12 months (P < 0.05; for both). One-year glycemic target response was inversely related to baseline HbA1c levels and diabetes duration among males, while metformin therapy (compared to oral combination therapy) was a significant predictor of better glycemic targets among females. Homeostasis model assessment-B, measured in 117 patients, predicted hypoglycemic response only in women (P = 0.009). Target 1-year weight loss was predicted by longer diabetes duration among males and by lower baseline HbA1c among females. Finally, no significant difference between genders was noted as to gastrointestinal side effects after exenatide therapy.
Conclusion: According to this "real world" experience, predictors of glycemic control and body weight loss after 12 months of exenatide BID therapy are different between genders in type 2 diabetes.
Keywords: GLP-1 agonist therapy; exenatide BID; real world setting; type 2 diabetes.
Figures
Similar articles
-
Efficacy and safety of biphasic insulin aspart 70/30 versus exenatide in subjects with type 2 diabetes failing to achieve glycemic control with metformin and a sulfonylurea.Curr Med Res Opin. 2009 Jan;25(1):65-75. doi: 10.1185/03007990802597951. Curr Med Res Opin. 2009. PMID: 19210140 Clinical Trial.
-
Exenatide once weekly plus dapagliflozin once daily versus exenatide or dapagliflozin alone in patients with type 2 diabetes inadequately controlled with metformin monotherapy (DURATION-8): a 28 week, multicentre, double-blind, phase 3, randomised controlled trial.Lancet Diabetes Endocrinol. 2016 Dec;4(12):1004-1016. doi: 10.1016/S2213-8587(16)30267-4. Epub 2016 Sep 16. Lancet Diabetes Endocrinol. 2016. PMID: 27651331 Clinical Trial.
-
Treatment Patterns and Persistence With GLP-1 RA Treatments Among Patients With Type 2 Diabetes in France: A Retrospective Cohort Analysis.Diabetes Ther. 2021 May;12(5):1553-1567. doi: 10.1007/s13300-021-01055-5. Epub 2021 Apr 17. Diabetes Ther. 2021. PMID: 33864629 Free PMC article.
-
Glycaemic efficacy of glucagon-like peptide-1 receptor agonists and dipeptidyl peptidase-4 inhibitors as add-on therapy to metformin in subjects with type 2 diabetes-a review and meta analysis.Diabetes Obes Metab. 2012 Aug;14(8):762-7. doi: 10.1111/j.1463-1326.2012.01603.x. Epub 2012 Apr 24. Diabetes Obes Metab. 2012. PMID: 22471248 Review.
-
Repaglinide : a pharmacoeconomic review of its use in type 2 diabetes mellitus.Pharmacoeconomics. 2004;22(6):389-411. doi: 10.2165/00019053-200422060-00005. Pharmacoeconomics. 2004. PMID: 15099124 Review.
Cited by
-
Analysis of patient-specific factors contributing to effectiveness of glucagon-like peptide-1 receptor agonists.J Am Pharm Assoc (2003). 2020 Nov-Dec;60(6):937-942. doi: 10.1016/j.japh.2020.06.023. Epub 2020 Aug 8. J Am Pharm Assoc (2003). 2020. PMID: 32778515 Free PMC article.
-
Determining predictors of early response to exenatide in patients with type 2 diabetes mellitus.J Diabetes Res. 2015;2015:162718. doi: 10.1155/2015/162718. Epub 2015 Jan 20. J Diabetes Res. 2015. PMID: 25688374 Free PMC article.
-
Marked weight loss on liraglutide 3.0 mg: Real-life experience of a Swiss cohort with obesity.Obesity (Silver Spring). 2023 Jan;31(1):74-82. doi: 10.1002/oby.23596. Epub 2022 Dec 7. Obesity (Silver Spring). 2023. PMID: 36478514 Free PMC article.
-
Treatment effect heterogeneity following type 2 diabetes treatment with GLP1-receptor agonists and SGLT2-inhibitors: a systematic review.Commun Med (Lond). 2023 Oct 5;3(1):131. doi: 10.1038/s43856-023-00359-w. Commun Med (Lond). 2023. PMID: 37794166 Free PMC article.
-
Clinical Effectiveness and Safety of Once-Weekly GLP-1 Receptor Agonist Dulaglutide as Add-On to Metformin or Metformin Plus Insulin Secretagogues in Obesity and Type 2 Diabetes.J Clin Med. 2021 Mar 2;10(5):985. doi: 10.3390/jcm10050985. J Clin Med. 2021. PMID: 33801192 Free PMC article.
References
-
- Turner R, Cull C, Holman R. United Kingdom Prospective Diabetes Study 17: a 9-year update of a randomized, controlled trial on the effect of improved metabolic control on complications in non-insulin-dependent diabetes mellitus. Ann Intern Med. 1996;124(1 Pt 2):136–145. - PubMed
-
- Stettler C, Allemann S, Jüni P, et al. Glycemic control and macrovascular disease in types 1 and 2 diabetes mellitus: Meta-analysis of randomized trials. Am Heart J. 2006;152(1):27–38. - PubMed
-
- Alvarez Guisasola F, Tofé Povedano S, Krishnarajah G, Lyu R, Mavros P, Yin D. Hypoglycaemic symptoms, treatment satisfaction, adherence and their associations with glycaemic goal in patients with type 2 diabetes mellitus: findings from the Real-Life Effectiveness and Care Patterns of Diabetes Management (RECAP-DM) Study. Diabetes Obes Metab. 2008;10( Suppl 1):25–32. - PubMed
-
- Weber KK, Lohmann T, Busch K, Donati-Hirsch I, Riel R. High frequency of unrecognized hypoglycaemias in patients with type 2 diabetes is discovered by continuous glucose monitoring. Exp Clin Endocrinol Diabetes. 2007;115(8):491–494. - PubMed
-
- Inzucchi SE, Bergenstal RM, Buse JB, et al. for American Diabetes Association (ADA), European Association for the Study of Diabetes (EASD) Management of hyperglycemia in type 2 diabetes: a patient-centered approach: position statement of the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD) Diabetes Care. 2012;35(6):1364–1379. - PMC - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources

