Inhibition of dengue virus entry into target cells using synthetic antiviral peptides
- PMID: 23630436
- PMCID: PMC3638295
- DOI: 10.7150/ijms.5037
Inhibition of dengue virus entry into target cells using synthetic antiviral peptides
Abstract
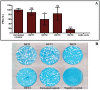
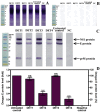
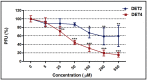
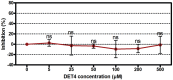
Despite the importance of DENV as a human pathogen, there is no specific treatment or protective vaccine. Successful entry into the host cells is necessary for establishing the infection. Recently, the virus entry step has become an attractive therapeutic strategy because it represents a barrier to suppress the onset of the infection. Four putative antiviral peptides were designed to target domain III of DENV-2 E protein using BioMoDroid algorithm. Two peptides showed significant inhibition of DENV when simultaneously incubated as shown by plaque formation assay, RT-qPCR, and Western blot analysis. Both DET4 and DET2 showed significant inhibition of virus entry (84.6% and 40.6% respectively) using micromolar concentrations. Furthermore, the TEM images showed that the inhibitory peptides caused structural abnormalities and alteration of the arrangement of the viral E protein, which interferes with virus binding and entry. Inhibition of DENV entry during the initial stages of infection can potentially reduce the viremia in infected humans resulting in prevention of the progression of dengue fever to the severe life-threatening infection, reduce the infected vector numbers, and thus break the transmission cycle. Moreover these peptides though designed against the conserved region in DENV-2 would have the potential to be active against all the serotypes of dengue and might be considered as Hits to begin designing and developing of more potent analogous peptides that could constitute as promising therapeutic agents for attenuating dengue infection.
Keywords: Antiviral peptides; Dengue virus; Domain III; Envelope protein; Inhibitory Peptides; Viral entry.
Conflict of interest statement
Competing Interests: The authors have declared that no competing interest exists.
Figures



References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

