Cerebral low-molecular metabolites influenced by intestinal microbiota: a pilot study
- PMID: 23630473
- PMCID: PMC3632785
- DOI: 10.3389/fnsys.2013.00009
Cerebral low-molecular metabolites influenced by intestinal microbiota: a pilot study
Abstract
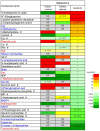
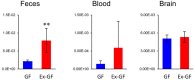
Recent studies suggest that intestinal microbiota influences gut-brain communication. In this study, we aimed to clarify the influence of intestinal microbiota on cerebral metabolism. We analyzed the cerebral metabolome of germ-free (GF) mice and Ex-GF mice, which were inoculated with suspension of feces obtained from specific pathogen-free mice, using capillary electrophoresis with time-of-flight mass spectrometry (CE-TOFMS). CE-TOFMS identified 196 metabolites from the cerebral metabolome in both GF and Ex-GF mice. The concentrations of 38 metabolites differed significantly (p < 0.05) between GF and Ex-GF mice. Approximately 10 of these metabolites are known to be involved in brain function, whilst the functions of the remainder are unclear. Furthermore, we observed a novel association between cerebral glycolytic metabolism and intestinal microbiota. Our work shows that cerebral metabolites are influenced by normal intestinal microbiota through the microbiota-gut-brain axis, and indicates that normal intestinal microbiota closely connected with brain health and disease, development, attenuation, learning, memory, and behavior.
Keywords: cerebrum; gut-brain axis; intestinal microbiota; metabolome; neurotransmitter.
Figures






References
-
- Carlson S. E., House S. G. (1986). Oral and intraperitoneal administration of N-acetylneuraminic acid: effect on rat cerebral and cerebellar N-acetylneuraminic acid. J. Nutr. 116, 881–886 - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources

