Evidence that adrenergic ventrolateral medullary cells are activated whereas precerebellar lateral reticular nucleus neurons are suppressed during REM sleep
- PMID: 23630631
- PMCID: PMC3632524
- DOI: 10.1371/journal.pone.0062410
Evidence that adrenergic ventrolateral medullary cells are activated whereas precerebellar lateral reticular nucleus neurons are suppressed during REM sleep
Abstract
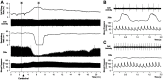
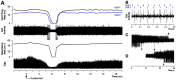
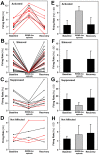
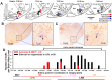
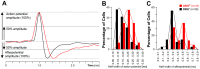
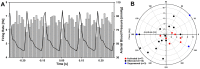
Rapid eye movement sleep (REMS) is generated in the brainstem by a distributed network of neurochemically distinct neurons. In the pons, the main subtypes are cholinergic and glutamatergic REMS-on cells and aminergic REMS-off cells. Pontine REMS-on cells send axons to the ventrolateral medulla (VLM), but little is known about REMS-related activity of VLM cells. In urethane-anesthetized rats, dorsomedial pontine injections of carbachol trigger REMS-like episodes that include cortical and hippocampal activation and suppression of motoneuronal activity; the episodes last 4-8 min and can be elicited repeatedly. We used this model to determine whether VLM catecholaminergic cells are silenced during REMS, as is typical of most aminergic neurons studied to date, and to investigate other REMS-related cells in this region. In 18 anesthetized, paralyzed and artificially ventilated rats, we obtained extracellular recordings from VLM cells when REMS-like episodes were elicited by pontine carbachol injections (10 mM, 10 nl). One major group were the cells that were activated during the episodes (n = 10). Their baseline firing rate of 3.7±2.1 (SD) Hz increased to 9.7±2.1 Hz. Most were found in the adrenergic C1 region and at sites located less than 50 µm from dopamine β-hydroxylase-positive (DBH(+)) neurons. Another major group were the silenced or suppressed cells (n = 35). Most were localized in the lateral reticular nucleus (LRN) and distantly from any DBH(+) cells. Their baseline firing rates were 6.8±4.4 Hz and 15.8±7.1 Hz, respectively, with the activity of the latter reduced to 7.4±3.8 Hz. We conclude that, in contrast to the pontine noradrenergic cells that are silenced during REMS, medullary adrenergic C1 neurons, many of which drive the sympathetic output, are activated. Our data also show that afferent input transmitted to the cerebellum through the LRN is attenuated during REMS. This may distort the spatial representation of body position during REMS.
Conflict of interest statement
Figures
Similar articles
-
Fos expression in pontomedullary catecholaminergic cells following rapid eye movement sleep-like episodes elicited by pontine carbachol in urethane-anesthetized rats.Neuroscience. 2008 Mar 3;152(1):208-22. doi: 10.1016/j.neuroscience.2007.11.013. Neuroscience. 2008. PMID: 18155849 Free PMC article.
-
A5 cells are silenced when REM sleep-like signs are elicited by pontine carbachol.J Appl Physiol (1985). 2002 Oct;93(4):1448-56. doi: 10.1152/japplphysiol.00225.2002. J Appl Physiol (1985). 2002. PMID: 12235046
-
Carbachol injections into the ventral pontine reticular formation activate locus coeruleus cells in urethane-anesthetized rats.Sleep. 2005 May;28(5):551-9. doi: 10.1093/sleep/28.5.551. Sleep. 2005. PMID: 16171268
-
Carbachol models of REM sleep: recent developments and new directions.Arch Ital Biol. 2001 Feb;139(1-2):147-68. Arch Ital Biol. 2001. PMID: 11256182 Review.
-
Locus coeruleus and dorsal pontine reticular influences on the gain of vestibulospinal reflexes.Prog Brain Res. 1991;88:435-62. doi: 10.1016/s0079-6123(08)63827-3. Prog Brain Res. 1991. PMID: 1813929 Review.
Cited by
-
Neural Control of the Upper Airway: Respiratory and State-Dependent Mechanisms.Compr Physiol. 2016 Sep 15;6(4):1801-1850. doi: 10.1002/cphy.c160002. Compr Physiol. 2016. PMID: 27783860 Free PMC article. Review.
-
The α1 adrenoceptor antagonist prazosin enhances sleep continuity in fear-conditioned Wistar-Kyoto rats.Prog Neuropsychopharmacol Biol Psychiatry. 2014 Mar 3;49:7-15. doi: 10.1016/j.pnpbp.2013.11.004. Epub 2013 Nov 15. Prog Neuropsychopharmacol Biol Psychiatry. 2014. PMID: 24246572 Free PMC article.
-
The anatomical, cellular and synaptic basis of motor atonia during rapid eye movement sleep.J Physiol. 2016 Oct 1;594(19):5391-414. doi: 10.1113/JP271324. Epub 2016 Jul 3. J Physiol. 2016. PMID: 27060683 Free PMC article. Review.
-
Connection Input Mapping and 3D Reconstruction of the Brainstem and Spinal Cord Projections to the CSF-Contacting Nucleus.Front Neural Circuits. 2020 Mar 31;14:11. doi: 10.3389/fncir.2020.00011. eCollection 2020. Front Neural Circuits. 2020. PMID: 32296310 Free PMC article.
-
Sleep-wake control of the upper airway by noradrenergic neurons, with and without intermittent hypoxia.Prog Brain Res. 2014;209:255-74. doi: 10.1016/B978-0-444-63274-6.00013-8. Prog Brain Res. 2014. PMID: 24746052 Free PMC article.
References
-
- Aserinsky E, Kleitman N (1953) Regularly occurring periods of eye motility, and concomitant phenomena, during sleep. Science 118: 273–274. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

