Borrelia burgdorferi bba66 gene inactivation results in attenuated mouse infection by tick transmission
- PMID: 23630963
- PMCID: PMC3697616
- DOI: 10.1128/IAI.00140-13
Borrelia burgdorferi bba66 gene inactivation results in attenuated mouse infection by tick transmission
Abstract
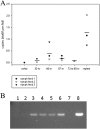
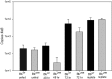
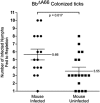
The impact of the Borrelia burgdorferi surface-localized immunogenic lipoprotein BBA66 on vector and host infection was evaluated by inactivating the encoding gene, bba66, and characterizing the mutant phenotype throughout the natural mouse-tick-mouse cycle. The BBA66-deficient mutant isolate, Bb(ΔA66), remained infectious in mice by needle inoculation of cultured organisms, but differences in spirochete burden and pathology in the tibiotarsal joint were observed relative to the parental wild-type (WT) strain. Ixodes scapularis larvae successfully acquired Bb(ΔA66) following feeding on infected mice, and the organisms persisted in these ticks through the molt to nymphs. A series of tick transmission experiments (n = 7) demonstrated that the ability of Bb(ΔA66)-infected nymphs to infect laboratory mice was significantly impaired compared to that of mice fed upon by WT-infected ticks. trans-complementation of Bb(ΔA66) with an intact copy of bba66 restored the WT infectious phenotype in mice via tick transmission. These results suggest a role for BBA66 in facilitating B. burgdorferi dissemination and transmission from the tick vector to the mammalian host as part of the disease process for Lyme borreliosis.
Figures




Similar articles
-
Adaptive immunity in Mus musculus influences the acquisition and abundance of Borrelia burgdorferi in Ixodes scapularis ticks.Appl Environ Microbiol. 2024 Dec 18;90(12):e0129924. doi: 10.1128/aem.01299-24. Epub 2024 Nov 6. Appl Environ Microbiol. 2024. PMID: 39503497 Free PMC article.
-
Assessment of decorin-binding protein A to the infectivity of Borrelia burgdorferi in the murine models of needle and tick infection.BMC Microbiol. 2008 May 28;8:82. doi: 10.1186/1471-2180-8-82. BMC Microbiol. 2008. PMID: 18507835 Free PMC article.
-
Passage through Ixodes scapularis ticks enhances the virulence of a weakly pathogenic isolate of Borrelia burgdorferi.Infect Immun. 2010 Jan;78(1):138-44. doi: 10.1128/IAI.00470-09. Epub 2009 Oct 12. Infect Immun. 2010. PMID: 19822652 Free PMC article.
-
Tick-host-pathogen interactions in Lyme borreliosis.Trends Parasitol. 2007 Sep;23(9):434-8. doi: 10.1016/j.pt.2007.07.001. Epub 2007 Jul 25. Trends Parasitol. 2007. PMID: 17656156 Review.
-
Borrelia burgdorferi--traveling incognito?Microbes Infect. 2006 Apr;8(5):1390-9. doi: 10.1016/j.micinf.2005.12.022. Epub 2006 Mar 24. Microbes Infect. 2006. PMID: 16698304 Review.
Cited by
-
The BB0345 Hypothetical Protein of Borrelia burgdorferi Is Essential for Mammalian Infection.Infect Immun. 2020 Nov 16;88(12):e00472-20. doi: 10.1128/IAI.00472-20. Print 2020 Nov 16. Infect Immun. 2020. PMID: 32928963 Free PMC article.
-
Human neuroglial cells internalize Borrelia burgdorferi by coiling phagocytosis mediated by Daam1.PLoS One. 2018 May 10;13(5):e0197413. doi: 10.1371/journal.pone.0197413. eCollection 2018. PLoS One. 2018. PMID: 29746581 Free PMC article.
-
The intergenic small non-coding RNA ittA is required for optimal infectivity and tissue tropism in Borrelia burgdorferi.PLoS Pathog. 2020 May 4;16(5):e1008423. doi: 10.1371/journal.ppat.1008423. eCollection 2020 May. PLoS Pathog. 2020. PMID: 32365143 Free PMC article.
-
Temperature-dependent sRNA transcriptome of the Lyme disease spirochete.BMC Genomics. 2017 Jan 5;18(1):28. doi: 10.1186/s12864-016-3398-3. BMC Genomics. 2017. PMID: 28056764 Free PMC article.
-
Structural and Functional Analysis of BBA03, Borrelia burgdorferi Competitive Advantage Promoting Outer Surface Lipoprotein.Pathogens. 2020 Oct 9;9(10):826. doi: 10.3390/pathogens9100826. Pathogens. 2020. PMID: 33050189 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

