Cytotoxicity of quantum dots and graphene oxide to erythroid cells and macrophages
- PMID: 23631472
- PMCID: PMC3646675
- DOI: 10.1186/1556-276X-8-198
Cytotoxicity of quantum dots and graphene oxide to erythroid cells and macrophages
Abstract
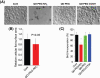
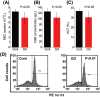
Great concerns have been raised about the exposure and possible adverse influence of nanomaterials due to their wide applications in a variety of fields, such as biomedicine and daily lives. The blood circulation system and blood cells form an important barrier against invaders, including nanomaterials. However, studies of the biological effects of nanomaterials on blood cells have been limited and without clear conclusions thus far. In the current study, the biological influence of quantum dots (QDs) with various surface coating on erythroid cells and graphene oxide (GO) on macrophages was closely investigated. We found that QDs posed great damage to macrophages through intracellular accumulation of QDs coupled with reactive oxygen species generation, particularly for QDs coated with PEG-NH2. QD modified with polyethylene glycol-conjugated amine particles exerted robust inhibition on cell proliferation of J744A.1 macrophages, irrespective of apoptosis. Additionally, to the best of our knowledge, our study is the first to have demonstrated that GO could provoke apoptosis of erythroid cells through oxidative stress in E14.5 fetal liver erythroid cells and in vivo administration of GO-diminished erythroid population in spleen, associated with disordered erythropoiesis in mice.
Figures





References
-
- Yong KT, Law WC, Hu R, Ye L, Liu L, Swihart MT, Prasad PN. Nanotoxicity assessment of quantum dots: from cellular to primate studies. Chem Soc Rev. 2012;8(3):1236–1250. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources

