Cyclohexa-2,5-diene-1,4-dione-based antiproliferative agents: design, synthesis, and cytotoxic evaluation
- PMID: 23631805
- PMCID: PMC3666920
- DOI: 10.1186/1756-9966-32-24
Cyclohexa-2,5-diene-1,4-dione-based antiproliferative agents: design, synthesis, and cytotoxic evaluation
Abstract
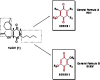
Background: Tumors are diseases characterized by uncontrolled cell growth and, in spite of the progress of medicine over the years, continue to represent a major threat to the health, requiring new therapies. Several synthetic compounds, such as those derived from natural sources, have been identified as anticancer drugs; among these compounds quinone represent the second largest class of anticancer agents in use. Several studies have shown that these act on tumor cells through several mechanisms. An important objective of this work is to develop quinoidscompounds showing antitumor activity, but with fewer side effects. The parachinone cannabinol HU-331, is a small molecule that with its core 4-hydroxy-1,4-benzoquinone, exhibits a potent and selective cytotoxic activity on different tumor cell lines. A series of derivatives 3-hydroxy-1,4-benzochinoni were thus developed through HU-331 chemical modifications. The purpose of the work is to test the ability of the compounds to induce proliferative inhibition and study the mechanisms of cell death.
Methods: The antitumor activities were evaluated in vitro by examining their cytotoxic effects against different human cancer cell lines. All cell lines tested were plated in 96-multiwell and treated with HU-100-V at different concentrations and cell viability was evaluated byMTT assay. Subsequently via flow cytometry (FACS) it was possible to assess apoptosis by the system of double labeling with PI and Annexin-V, and the effect of the compounds on ROS formation by measuring the dichlorofluorescein fluorescence.
Results: The substitution by n-hexyl chain considerably enhanced the bioactivity of the compounds. In details, 2-hexyl-5-hydroxycyclohexa-2,5-diene-1,4-dione (V), 2,5-Dimethoxy-3-hexyl-2,5-cyclohexadiene-1,4-dione (XII) and 2-hydroxy-5-methoxy-3-hexyl-cyclohexa-2,5-diene-1,4-dione (XIII) showed most prominent cytotoxicity against almost human tumour cell lines. Compound V was further subjected to downstream apoptotic analysis, demostrating a time-dependent pro-apoptotic activity on human melanoma M14 cell line mediated by caspases activation and poly-(ADP-ribose)-polymerase (PARP) protein cleavage.
Conclusions: These findings indicate that 2-hexyl-5-idrossicicloesa-2,5-diene-1,4-dione can be a promising compound for the design of a new class of antineoplastic derivatives.Carmen Petronzi, Michela Festa, Antonella Peduto and Maria Castellano: equally contributed equally to this work.
Figures




References
-
- Yu CC, Wu PJ, Hsu JL, Ho YF, Hsu LC, Chang YJ, Chang HS, Chen IS, Guh JH. Ardisianone, a natural benzoquinone, efficiently induces apoptosis in human hormone-refractory prostate cancers through mitochondrial damage stress and survivin downregulation. Prostate. 2013;73(2):133–145. doi: 10.1002/pros.22548. Epub 2012 Jun 5.2012. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

