Reactive intermediates in cytochrome p450 catalysis
- PMID: 23632017
- PMCID: PMC3682513
- DOI: 10.1074/jbc.R113.473108
Reactive intermediates in cytochrome p450 catalysis
Abstract
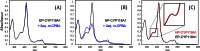
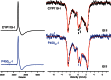
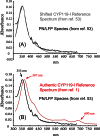
Recently, we reported the spectroscopic and kinetic characterizations of cytochrome P450 compound I in CYP119A1, effectively closing the catalytic cycle of cytochrome P450-mediated hydroxylations. In this minireview, we focus on the developments that made this breakthrough possible. We examine the importance of enzyme purification in the quest for reactive intermediates and report the preparation of compound I in a second P450 (P450ST). In an effort to bring clarity to the field, we also examine the validity of controversial reports claiming the production of P450 compound I through the use of peroxynitrite and laser flash photolysis.
Keywords: Cytochrome P450; Enzyme Catalysis; Enzyme Purification; Heme; P450 Compound I; Spectroscopy.
Figures
References
-
- Rittle J., Green M. T. (2010) Cytochrome P450 compound I: capture, characterization, and C–H bond activation kinetics. Science 330, 933–937 - PubMed
-
- Moss T. H., Ehrenberg A., Bearden A. J. (1969) Mössbauer spectroscopic evidence for electronic configuration of iron in horseradish peroxidase and its peroxide derivatives. Biochemistry 8, 4159–4162 - PubMed
-
- Schonbaum G. R., Lo S. (1972) Interaction of peroxidases with aromatic peracids and alkyl peroxides. Product analysis. J. Biol. Chem. 247, 3353–3360 - PubMed
-
- Jung C. (2011) The mystery of cytochrome P450 compound I: a mini-review dedicated to Klaus Ruckpaul. Biochim. Biophys. Acta 1814, 46–57 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

