Investigations of the contribution of a putative glycine hinge to ryanodine receptor channel gating
- PMID: 23632022
- PMCID: PMC3675601
- DOI: 10.1074/jbc.M113.465310
Investigations of the contribution of a putative glycine hinge to ryanodine receptor channel gating
Abstract
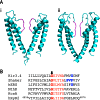
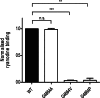
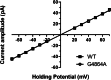
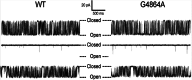
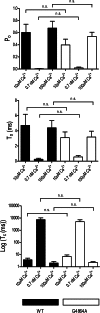
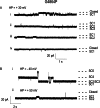
Ryanodine receptor channels (RyR) are key components of striated muscle excitation-contraction coupling, and alterations in their function underlie both inherited and acquired disease. A full understanding of the disease process will require a detailed knowledge of the mechanisms and structures involved in RyR function. Unfortunately, high-resolution structural data, such as exist for K(+)-selective channels, are not available for RyR. In the absence of these data, we have used modeling to identify similarities in the structural elements of K(+) channel pore-forming regions and postulated equivalent regions of RyR. This has identified a sequence of residues in the cytosolic cavity-lining transmembrane helix of RyR (G(4864)LIIDA(4869) in RyR2) analogous to the glycine hinge motif present in many K(+) channels. Gating in these K(+) channels can be disrupted by substitution of residues for the hinge glycine. We investigated the involvement of glycine 4864 in RyR2 gating by monitoring properties of recombinant human RyR2 channels in which this glycine is replaced by residues that alter gating in K(+) channels. Our data demonstrate that introducing alanine at position 4864 produces no significant change in RyR2 function. In contrast, function is altered when glycine 4864 is replaced by either valine or proline, the former preventing channel opening and the latter modifying both ion translocation and gating. Our studies reveal novel information on the structural basis of RyR gating, identifying both similarities with, and differences from, K(+) channels. Glycine 4864 is not absolutely required for channel gating, but some flexibility at this point in the cavity-lining transmembrane helix is necessary for normal RyR function.
Keywords: Calcium Channels; Calcium Intracellular Release; Glycine Hinge; Ion Channels; Ryanodine Receptor; Sarcoplasmic Reticulum (SR); Single Channel Gating.
Figures


Similar articles
-
Channel Gating Dependence on Pore Lining Helix Glycine Residues in Skeletal Muscle Ryanodine Receptor.J Biol Chem. 2015 Jul 10;290(28):17535-45. doi: 10.1074/jbc.M115.659672. Epub 2015 May 21. J Biol Chem. 2015. PMID: 25998124 Free PMC article.
-
The contribution of hydrophobic residues in the pore-forming region of the ryanodine receptor channel to block by large tetraalkylammonium cations and Shaker B inactivation peptides.J Gen Physiol. 2012 Sep;140(3):325-39. doi: 10.1085/jgp.201210851. J Gen Physiol. 2012. PMID: 22930804 Free PMC article.
-
The Cytoplasmic Region of Inner Helix S6 Is an Important Determinant of Cardiac Ryanodine Receptor Channel Gating.J Biol Chem. 2016 Dec 9;291(50):26024-26034. doi: 10.1074/jbc.M116.758821. Epub 2016 Oct 27. J Biol Chem. 2016. PMID: 27789712 Free PMC article.
-
Light at the end of the Ca(2+)-release channel tunnel: structures and mechanisms involved in ion translocation in ryanodine receptor channels.Q Rev Biophys. 2001 Feb;34(1):61-104. doi: 10.1017/s0033583501003675. Q Rev Biophys. 2001. PMID: 11388090 Review.
-
Immunophilins and coupled gating of ryanodine receptors.Curr Top Med Chem. 2003;3(12):1383-91. doi: 10.2174/1568026033451907. Curr Top Med Chem. 2003. PMID: 12871170 Review.
Cited by
-
Structural insights into Ca(2+)-activated long-range allosteric channel gating of RyR1.Cell Res. 2016 Sep;26(9):977-94. doi: 10.1038/cr.2016.99. Epub 2016 Aug 30. Cell Res. 2016. PMID: 27573175 Free PMC article.
-
G4941K substitution in the pore-lining S6 helix of the skeletal muscle ryanodine receptor increases RyR1 sensitivity to cytosolic and luminal Ca2.J Biol Chem. 2018 Feb 9;293(6):2015-2028. doi: 10.1074/jbc.M117.803247. Epub 2017 Dec 18. J Biol Chem. 2018. PMID: 29255089 Free PMC article.
-
Structural and Mechanistic Insights into the Regulation of the Fundamental Rho Regulator RhoGDIα by Lysine Acetylation.J Biol Chem. 2016 Mar 11;291(11):5484-5499. doi: 10.1074/jbc.M115.707091. Epub 2015 Dec 30. J Biol Chem. 2016. PMID: 26719334 Free PMC article.
-
Identification of avoidance genes through neural pathway-specific forward optogenetics.PLoS Genet. 2019 Dec 31;15(12):e1008509. doi: 10.1371/journal.pgen.1008509. eCollection 2019 Dec. PLoS Genet. 2019. PMID: 31891575 Free PMC article.
-
Channel Gating Dependence on Pore Lining Helix Glycine Residues in Skeletal Muscle Ryanodine Receptor.J Biol Chem. 2015 Jul 10;290(28):17535-45. doi: 10.1074/jbc.M115.659672. Epub 2015 May 21. J Biol Chem. 2015. PMID: 25998124 Free PMC article.
References
-
- Bers D. M. (2002) Cardiac excitation-contraction coupling. Nature 415, 198–205 - PubMed
-
- Thomas L. N., Williams A. J. (2012) Pharmacology of ryanodine receptors and Ca2+-induced Ca2+ release. Wiley Interdiscip. Rev. Membr. Transp. Signal. 1, 383–397
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

