Synthesis of ¹⁸O-labeled RNA for application to kinetic studies and imaging
- PMID: 23632164
- PMCID: PMC3695515
- DOI: 10.1093/nar/gkt344
Synthesis of ¹⁸O-labeled RNA for application to kinetic studies and imaging
Abstract
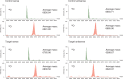
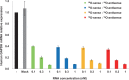
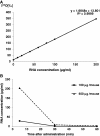
Radioisotopes and fluorescent compounds are frequently used for RNA labeling but are unsuitable for clinical studies of RNA drugs because of the risk from radiation exposure or the nonequivalence arising from covalently attached fluorophores. Here, we report a practical phosphoramidite solid-phase synthesis of (18)O-labeled RNA that avoids these disadvantages, and we demonstrate its application to quantification and imaging. The synthesis involves the introduction of a nonbridging (18)O atom into the phosphate group during the oxidation step of the synthetic cycle by using (18)O water as the oxygen donor. The (18)O label in the RNA was stable at pH 3-8.5, while the physicochemical and biological properties of labeled and unlabeled short interfering RNA were indistinguishable by circular dichroism, melting temperature and RNA-interference activity. The (18)O/(16)O ratio as measured by isotope ratio mass spectrometry increased linearly with the concentration of (18)O-labeled RNA, and this technique was used to determine the blood concentration of (18)O-labeled RNA after administration to mice. (18)O-labeled RNA transfected into human A549 cells was visualized by isotope microscopy. The RNA was observed in foci in the cytoplasm around the nucleus, presumably corresponding to endosomes. These methodologies may be useful for kinetic and cellular-localization studies of RNA in basic and pharmaceutical studies.
Figures



Similar articles
-
Regression analysis for comparing protein samples with 16O/18O stable-isotope labeled mass spectrometry.Bioinformatics. 2006 Nov 15;22(22):2739-45. doi: 10.1093/bioinformatics/btl464. Epub 2006 Sep 5. Bioinformatics. 2006. PMID: 16954138
-
Trypsin catalyzed 16O-to-18O exchange for comparative proteomics: tandem mass spectrometry comparison using MALDI-TOF, ESI-QTOF, and ESI-ion trap mass spectrometers.J Am Soc Mass Spectrom. 2003 Jul;14(7):704-18. doi: 10.1016/S1044-0305(03)00207-1. J Am Soc Mass Spectrom. 2003. PMID: 12837592
-
Measuring proteome dynamics in vivo: as easy as adding water?Mol Cell Proteomics. 2009 Dec;8(12):2653-63. doi: 10.1074/mcp.M900026-MCP200. Epub 2009 Sep 1. Mol Cell Proteomics. 2009. PMID: 19724074 Free PMC article.
-
The use of isotope effects to determine enzyme mechanisms.Arch Biochem Biophys. 2005 Jan 1;433(1):2-12. doi: 10.1016/j.abb.2004.08.027. Arch Biochem Biophys. 2005. PMID: 15581561 Review.
-
Heavy atom labeled nucleotides for measurement of kinetic isotope effects.Biochim Biophys Acta. 2015 Nov;1854(11):1737-45. doi: 10.1016/j.bbapap.2015.03.007. Epub 2015 Mar 27. Biochim Biophys Acta. 2015. PMID: 25828952 Free PMC article. Review.
Cited by
-
Stable Isotope Phosphate Labelling of Diverse Metabolites is Enabled by a Family of 18O-Phosphoramidites.Angew Chem Weinheim Bergstr Ger. 2022 Jan 26;134(5):e202112457. doi: 10.1002/ange.202112457. Epub 2021 Nov 23. Angew Chem Weinheim Bergstr Ger. 2022. PMID: 38505299 Free PMC article.
-
Distribution of Antisense Oligonucleotides in Rat Eyeballs Using MALDI Imaging Mass Spectrometry.Mass Spectrom (Tokyo). 2018;7(1):A0070. doi: 10.5702/massspectrometry.A0070. Epub 2018 Sep 11. Mass Spectrom (Tokyo). 2018. PMID: 30214850 Free PMC article.
-
A general and efficient approach to synthesize the phosphoramidites of 5'-18O labeled purine nucleosides.Nucleosides Nucleotides Nucleic Acids. 2023;42(11):930-943. doi: 10.1080/15257770.2023.2218421. Epub 2023 May 26. Nucleosides Nucleotides Nucleic Acids. 2023. PMID: 37233721 Free PMC article.
-
Organogermanium suppresses cell death due to oxidative stress in normal human dermal fibroblasts.Sci Rep. 2019 Sep 20;9(1):13637. doi: 10.1038/s41598-019-49883-7. Sci Rep. 2019. PMID: 31541125 Free PMC article.
-
Stable Isotope Phosphate Labelling of Diverse Metabolites is Enabled by a Family of 18 O-Phosphoramidites.Angew Chem Int Ed Engl. 2022 Jan 26;61(5):e202112457. doi: 10.1002/anie.202112457. Epub 2021 Nov 23. Angew Chem Int Ed Engl. 2022. PMID: 34734451 Free PMC article.
References
-
- Fire A, Xu S, Montgomery MK, Kostas SA, Driver SE, Mello CC. Potent and specific genetic interference by double-stranded RNA in Caenorhabditis elegans. Nature. 1998;391:806–811. - PubMed
-
- Funatsu T, Harada Y, Tokunaga M, Saito K, Yanagida T. Imaging of single fluorescent molecules and individual ATP turnovers by single myosin molecules in aqueous solution. Nature. 1995;374:555–559. - PubMed
-
- Sechi S, Oda Y. Quantitative proteomics using mass spectrometry. Curr. Opin. Chem. Biol. 2003;7:70–77. - PubMed
-
- Yao X, Freas A, Ramirez J, Demirev PA, Fenselau C. Proteolytic 18O labeling for comparative proteomics: model studies with two serotypes of adenovirus. Anal. Chem. 2001;73:2836–2842. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

