Compartmentalized chemokine-dependent regulatory T-cell inhibition of allergic pulmonary inflammation
- PMID: 23632297
- PMCID: PMC3703653
- DOI: 10.1016/j.jaci.2013.03.002
Compartmentalized chemokine-dependent regulatory T-cell inhibition of allergic pulmonary inflammation
Abstract
Background: Induction of endogenous regulatory T (Treg) cells represents an exciting new potential modality for treating allergic diseases, such as asthma. Treg cells have been implicated in the regulation of asthma, but the anatomic location in which they exert their regulatory function and the mechanisms controlling the migration necessary for their suppressive function in asthma are not known. Understanding these aspects of Treg cell biology will be important for harnessing their power in the clinic.
Objective: We sought to determine the anatomic location at which Treg cells exert their regulatory function in the sensitization and effector phases of allergic asthma and to determine the chemokine receptors that control the migration of Treg cells to these sites in vivo in both mice and human subjects.
Methods: The clinical efficacy and anatomic location of adoptively transferred chemokine receptor-deficient CD4(+)CD25(+) forkhead box protein 3-positive Treg cells was determined in the sensitization and effector phases of allergic airway inflammation in mice. The chemokine receptor expression profile was determined on Treg cells recruited into the human airway after bronchoscopic segmental allergen challenge of asthmatic patients.
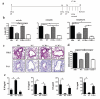
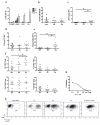
Results: We show that CCR7, but not CCR4, is required on Treg cells to suppress allergic airway inflammation during the sensitization phase. In contrast, CCR4, but not CCR7, is required on Treg cells to suppress allergic airway inflammation during the effector phase. Consistent with our murine studies, human subjects with allergic asthma had an increase in CCR4-expressing functional Treg cells in the lungs after segmental allergen challenge.
Conclusion: The location of Treg cell function differs during allergic sensitization and allergen-induced recall responses in the lung, and this differential localization is critically dependent on differential chemokine function.
Copyright © 2013 American Academy of Allergy, Asthma & Immunology. Published by Mosby, Inc. All rights reserved.
Figures


References
-
- Larche M, Robinson DS, Kay AB. The role of T lymphocytes in the pathogenesis of asthma. J Allergy Clin Immunol. 2003;111:450–63. quiz 64. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

