Association between efavirenz-based compared with nevirapine-based antiretroviral regimens and virological failure in HIV-infected children
- PMID: 23632724
- PMCID: PMC3748602
- DOI: 10.1001/jama.2013.3710
Association between efavirenz-based compared with nevirapine-based antiretroviral regimens and virological failure in HIV-infected children
Abstract
Importance: Worldwide, the nonnucleoside reverse transcriptase inhibitors (NNRTIs) efavirenz and nevirapine are commonly used in first-line antiretroviral regimens in both adults and children with human immunodeficiency virus (HIV) infection. Data on the comparative effectiveness of these medications in children are limited.
Objective: To investigate whether virological failure is more likely among children who initiated 1 or the other NNRTI-based HIV treatment.
Design, setting, and participants: Retrospective cohort study of children (aged 3-16 years) who initiated efavirenz-based (n = 421) or nevirapine-based (n = 383) treatment between April 2002 and January 2011 at a large pediatric HIV care setting in Botswana.
Main outcomes and measures: The primary outcome was time from initiation of therapy to virological failure. Virological failure was defined as lack of plasma HIV RNA suppression to less than 400 copies/mL by 6 months or confirmed HIV RNA of 400 copies/mL or greater after suppression. Cox proportional hazards regression analysis compared time to virological failure by regimen. Multivariable Cox regression controlled for age, sex, baseline immunologic category, baseline clinical category, baseline viral load, nutritional status, NRTIs used, receipt of single-dose nevirapine, and treatment for tuberculosis.
Results: With a median follow-up time of 69 months (range, 6-112 months; interquartile range, 23-87 months), 57 children (13.5%; 95% CI, 10.4%-17.2%) initiating treatment with efavirenz and 101 children (26.4%; 95% CI, 22.0%-31.1%) initiating treatment with nevirapine had virological failure. There were 11 children (2.6%; 95% CI, 1.3%-4.6%) receiving efavirenz and 20 children (5.2%; 95% CI, 3.2%-7.9%) receiving nevirapine who never achieved virological suppression. The Cox proportional hazard ratio for the combined virological failure end point was 2.0 (95% CI, 1.4-2.7; log rank P < .001, favoring efavirenz). None of the measured covariates affected the estimated hazard ratio in the multivariable analyses.
Conclusions and relevance: Among children aged 3 to 16 years infected with HIV and treated at a clinic in Botswana, the use of efavirenz compared with nevirapine as initial antiretroviral treatment was associated with less virological failure. These findings may warrant additional research evaluating the use of efavirenz and nevirapine for pediatric patients.
Figures


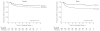
References
-
- UNAIDS [Accessed November 21, 2010];AIDS epidemic update. 2009 http://data.unaids.org/pub/Report/2009/JC1700_Epi_Update_2009_en.pdf.
-
- UNICEF [Accessibility verified March 28, 2013];Children and AIDS: fifth stocktaking report. 2010 http://www.unicef.org/publications/files/Children_and_AIDS-Fifth_Stockta....
-
- Bolton-Moore C, Mubiana-Mbewe M, Cantrell RA, et al. Clinical outcomes and CD4 cell response in children receiving antiretroviral therapy at primary health care facilities in Zambia. JAMA. 2007;298(16):1888–1899. - PubMed
-
- Little K, Thorne C, Luo C, et al. Disease progression in children with vertically-acquired HIV infection in sub-Saharan Africa: reviewing the need for HIV treatment. Curr HIV Res. 2007;5(2):139–153. - PubMed
-
- World Health Organization . Antiretroviral Therapy for HIV Infection in Infants and Children: Towards Universal Access: Recommendations for a Public Health Approach. World Health Organization Press; Geneva, Switzerland: 2010. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

