GM-CSF-dependent pSTAT5 sensitivity is a feature with therapeutic potential in chronic myelomonocytic leukemia
- PMID: 23632888
- PMCID: PMC4347259
- DOI: 10.1182/blood-2012-10-460170
GM-CSF-dependent pSTAT5 sensitivity is a feature with therapeutic potential in chronic myelomonocytic leukemia
Abstract
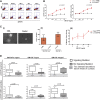
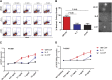
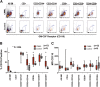
Granulocyte-macrophage-colony-stimulating factor (GM-CSF) hypersensitivity is a hallmark of juvenile myelomonocytic leukemia (JMML) but has not been systematically shown in the related human disease chronic myelomonocytic leukemia (CMML). We find that primary CMML samples demonstrate GM-CSF-dependent hypersensitivity by hematopoietic colony formation assays and phospho-STAT5 (pSTAT5) flow cytometry compared with healthy donors. Among CMML patients, the pSTAT5 hypersensitive response positively correlated with high-risk disease, peripheral leukocytes, monocytes, and signaling-associated mutations. When compared with IL-3 and G-CSF, GM-CSF hypersensitivity was cytokine specific and thus a possible target for intervention in CMML. To explore this possibility, we treated primary CMML cells with KB003, a novel monoclonal anti-GM-CSF antibody, and JAK2 inhibitors. We found that an elevated proportion of immature GM-CSF receptor-α(R) subunit-expressing cells were present in the bone marrow myeloid compartment of CMML. In survival assays, we found that myeloid and monocytic progenitors were sensitive to GM-CSF signal inhibition. Our data indicate that a committed myeloid precursor expressing CD38 may represent the progenitor population with enhanced GM-CSF dependence in CMML, consistent with results in JMML. These preclinical data indicate that GM-CSF signaling inhibitors merit further investigation in CMML and that GM-CSFR expression on myeloid progenitors may be a biomarker for this therapy.
Figures


References
-
- Emanuel PD. Juvenile myelomonocytic leukemia and chronic myelomonocytic leukemia. Leukemia. 2008;22(7):1335–1342. - PubMed
-
- Rollison DE, Howlader N, Smith MT, et al. Epidemiology of myelodysplastic syndromes and chronic myeloproliferative disorders in the United States, 2001-2004, using data from the NAACCR and SEER programs. Blood. 2008;112(1):45–52. - PubMed
-
- Kohlmann A, Grossmann V, Klein H-U, et al. Next-generation sequencing technology reveals a characteristic pattern of molecular mutations in 72.8% of chronic myelomonocytic leukemia by detecting frequent alterations in TET2, CBL, RAS, and RUNX1. J Clin Oncol. 2010;28(24):3858–3865. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

