Results of inotuzumab ozogamicin, a CD22 monoclonal antibody, in refractory and relapsed acute lymphocytic leukemia
- PMID: 23633004
- PMCID: PMC3720844
- DOI: 10.1002/cncr.28136
Results of inotuzumab ozogamicin, a CD22 monoclonal antibody, in refractory and relapsed acute lymphocytic leukemia
Abstract
Background: CD22 expression occurs in >90% of patients with acute lymphocytic leukemia (ALL). Inotuzumab ozogamicin, a CD22 monoclonal antibody bound to calicheamicin, is active in ALL.
Methods: Patients with refractory-relapsed ALL received treatment with inotuzumab. The first 49 patients received single-dose, intravenous inotuzumab at doses of 1.3 to 1.8 mg/m2 every 3 to 4 weeks. In the next 41 patients, the schedule was modified to inotuzumab weekly at a dose of 0.8 mg/m2 on day 1 and at a dose of 0.5 mg/m2 on days 8 and 15, every 3 to 4 weeks, based on higher in vitro efficacy with more frequent exposure.
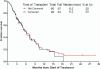
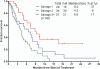
Results: Ninety patients were treated; 68% were in salvage 2 or beyond. Overall, 17 patients (19%) achieved a complete response (CR), 27 (30%) had a CR with no platelet recovery (CRp), and 8 (9%) had a bone marrow CR (no recovery of counts), for an overall response rate of 58%. Response rates were similar for single-dose and weekly dose inotuzumab (57% vs 59%, respectively). The median survival was 6.2 months overall, 5.0 months with the single-dose schedule, and 7.3 months with the weekly dose schedule. The median survival was 9.2 months for patients in salvage 1 (37% at 1 year), 4.3 months for patients in salvage 2, and 6.6 months for patients in salvage 3 or later. The median remission duration was 7 months. Reversible bilirubin elevation, fever, and hypotension were observed less frequently on the weekly dose. In total, 36 of 90 patients (40%) underwent allogeneic stem cell transplantation. Veno-occlusive disease was noted in 6 of 36 patients after stem cell transplantation (17%), was less frequent after the weekly schedule (7%), and with less alkylators in the preparative regimen.
Conclusions: Inotuzumab single-agent therapy was highly active, safe, and convenient in patients with refractory-relapsed ALL. A weekly dose schedule appeared to be equally effective and less toxic than a single-dose schedule.
Keywords: acute lymphocytic leukemia; inotuzumab; monoclonal antibody; refractory; relapsed.
© 2013 American Cancer Society.
Figures




Comment in
-
CD22 monoclonal antibody therapies in relapsed/refractory acute lymphoblastic leukemia.Cancer. 2013 Aug 1;119(15):2671-4. doi: 10.1002/cncr.28135. Epub 2013 Apr 30. Cancer. 2013. PMID: 23633448 No abstract available.
References
-
- Gokbuget N, Hoelzer D, Arnold R, et al. Treatment of adult ALL according to protocols of the German multicenter study group for adult ALL (GMALL). Hematol Oncol Clin North Am. 2000;14:1307–1325. - PubMed
-
- Bassan R, Hoelzer D. Modern therapy of acute lymphoblastic leukemia. Journ of Clin Onc. 2011;29(5):532–543. - PubMed
-
- Thomas DA, Faderl S, O'Brien S, et al. Chemoimmunotherapy with hyper-CVAD plus rituximab for the treatment of adult Burkitt and Burkitt-type lymphoma or acute lymphoblastic leukemia. Cancer. 2006;106:1569–1580. - PubMed
-
- Gokbuget N, Hoelzer D. Rituximab in the treatment of adult ALL. Ann Hematol. 2006;85:117–119.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

