Three-dimensional histological specimen preparation for accurate imaging and spatial reconstruction of the middle and inner ear
- PMID: 23633112
- PMCID: PMC3702969
- DOI: 10.1007/s11548-013-0825-7
Three-dimensional histological specimen preparation for accurate imaging and spatial reconstruction of the middle and inner ear
Abstract
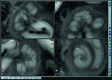
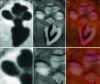
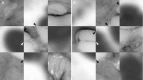
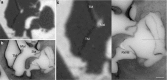
Purpose: This paper presents a highly accurate cross-sectional preparation technique. The research aim was to develop an adequate imaging modality for both soft and bony tissue structures featuring high contrast and high resolution. Therefore, the advancement of an already existing micro-grinding procedure was pursued. The central objectives were to preserve spatial relations and to ensure the accurate three-dimensional reconstruction of histological sections.
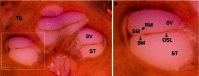
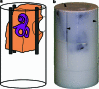
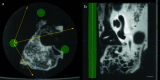
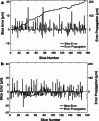
Methods: Twelve human temporal bone specimens including middle and inner ear structures were utilized. They were embedded in epoxy resin, then dissected by serial grinding and finally digitalized. The actual abrasion of each grinding slice was measured using a tactile length gauge with an accuracy of one micrometre. The cross-sectional images were aligned with the aid of artificial markers and by applying a feature-based, custom-made auto-registration algorithm. To determine the accuracy of the overall reconstruction procedure, a well-known reference object was used for comparison. To ensure the compatibility of the histological data with conventional clinical image data, the image stacks were finally converted into the DICOM standard.
Results: The image fusion of data from temporal bone specimens' and from non-destructive flat-panel-based volume computed tomography confirmed the spatial accuracy achieved by the procedure, as did the evaluation using the reference object.
Conclusion: This systematic and easy-to-follow preparation technique enables the three-dimensional (3D) histological reconstruction of complex soft and bony tissue structures. It facilitates the creation of detailed and spatially correct 3D anatomical models. Such models are of great benefit for image-based segmentation and planning in the field of computer-assisted surgery as well as in finite element analysis. In the context of human inner ear surgery, three-dimensional histology will improve the experimental evaluation and determination of intra-cochlear trauma after the insertion of an electrode array of a cochlear implant system.
Conflict of interest statement
The authors declare that they have no conflict of interest.
Figures







References
-
- Aerts J, Dirckx J, Dierick M, Van Hoorebeke L (2012) High-resolution 3D surface model of bone and soft tissue structures in the human middle ear. Assoc Res Otolaryngol Abstr 134, p 47
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

