Transfer RNA post-transcriptional processing, turnover, and subcellular dynamics in the yeast Saccharomyces cerevisiae
- PMID: 23633143
- PMCID: PMC3632480
- DOI: 10.1534/genetics.112.147470
Transfer RNA post-transcriptional processing, turnover, and subcellular dynamics in the yeast Saccharomyces cerevisiae
Abstract
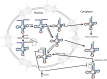
Transfer RNAs (tRNAs) are essential for protein synthesis. In eukaryotes, tRNA biosynthesis employs a specialized RNA polymerase that generates initial transcripts that must be subsequently altered via a multitude of post-transcriptional steps before the tRNAs beome mature molecules that function in protein synthesis. Genetic, genomic, biochemical, and cell biological approaches possible in the powerful Saccharomyces cerevisiae system have led to exciting advances in our understandings of tRNA post-transcriptional processing as well as to novel insights into tRNA turnover and tRNA subcellular dynamics. tRNA processing steps include removal of transcribed leader and trailer sequences, addition of CCA to the 3' mature sequence and, for tRNA(His), addition of a 5' G. About 20% of yeast tRNAs are encoded by intron-containing genes. The three-step splicing process to remove the introns surprisingly occurs in the cytoplasm in yeast and each of the splicing enzymes appears to moonlight in functions in addition to tRNA splicing. There are 25 different nucleoside modifications that are added post-transcriptionally, creating tRNAs in which ∼15% of the residues are nucleosides other than A, G, U, or C. These modified nucleosides serve numerous important functions including tRNA discrimination, translation fidelity, and tRNA quality control. Mature tRNAs are very stable, but nevertheless yeast cells possess multiple pathways to degrade inappropriately processed or folded tRNAs. Mature tRNAs are also dynamic in cells, moving from the cytoplasm to the nucleus and back again to the cytoplasm; the mechanism and function of this retrograde process is poorly understood. Here, the state of knowledge for tRNA post-transcriptional processing, turnover, and subcellular dynamics is addressed, highlighting the questions that remain.
Figures




References
-
- Abelson J., Trotta C. R., Li H., 1998. tRNA splicing. J. Biol. Chem. 273: 12685–12688. - PubMed
-
- Aebi M., Kirchner G., Chen J. Y., Vijayraghavan U., Jacobson A., et al. , 1990. Isolation of a temperature-sensitive mutant with an altered tRNA nucleotidyltransferase and cloning of the gene encoding tRNA nucleotidyltransferase in the yeast Saccharomyces cerevisiae. J. Biol. Chem. 265: 16216–16220. - PubMed
-
- Alexandrov A., Chernyakov I., Gu W., Hiley S. L., Hughes T. R., et al. , 2006. Rapid tRNA decay can result from lack of nonessential modifications. Mol. Cell 21: 87–96. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

