How stem cells speak with host immune cells in inflammatory brain diseases
- PMID: 23633288
- PMCID: PMC4063195
- DOI: 10.1002/glia.22500
How stem cells speak with host immune cells in inflammatory brain diseases
Abstract
Advances in stem cell biology have raised great expectations that diseases and injuries of the central nervous system (CNS) may be ameliorated by the development of non-hematopoietic stem cell medicines. Yet, the application of adult stem cells as CNS therapeutics is challenging and the interpretation of some of the outcomes ambiguous. In fact, the initial idea that stem cell transplants work only via structural cell replacement has been challenged by the observation of consistent cellular signaling between the graft and the host. Cellular signaling is the foundation of coordinated actions and flexible responses, and arises via networks of exchanging and interacting molecules that transmit patterns of information between cells. Sustained stem cell graft-to-host communication leads to remarkable trophic effects on endogenous brain cells and beneficial modulatory actions on innate and adaptive immune responses in vivo, ultimately promoting the healing of the injured CNS. Among a number of adult stem cell types, mesenchymal stem cells (MSCs) and neural stem/precursor cells (NPCs) are being extensively investigated for their ability to signal to the immune system upon transplantation in experimental CNS diseases. Here, we focus on the main cellular signaling pathways that grafted MSCs and NPCs use to establish a therapeutically relevant cross talk with host immune cells, while examining the role of inflammation in regulating some of the bidirectionality of these communications. We propose that the identification of the players involved in stem cell signaling might contribute to the development of innovative, high clinical impact therapeutics for inflammatory CNS diseases.
Keywords: immune modulation; inflammation; mesenchymal stem cells; neural stem cells; stem cell-host interactions.
Copyright © 2013 Wiley Periodicals, Inc.
Figures

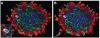

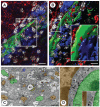
References
-
- Aboody K, Capela A, Niazi N, Stern JH, Temple S. Translating stem cell studies to the clinic for CNS repair: Current state of the art and the need for a Rosetta Stone. Neuron. 2011;70:597–613. - PubMed
-
- Aggarwal S, Pittenger MF. Human mesenchymal stem cells modulate allogeneic immune cell responses. Blood. 2005;105:1815–1822. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

