Skin patch and vaginal ring versus combined oral contraceptives for contraception
- PMID: 23633314
- PMCID: PMC7154336
- DOI: 10.1002/14651858.CD003552.pub4
Skin patch and vaginal ring versus combined oral contraceptives for contraception
Abstract
Background: The delivery of combination contraceptive steroids from a transdermal contraceptive patch or a contraceptive vaginal ring offers potential advantages over the traditional oral route. The transdermal patch and vaginal ring could require a lower dose due to increased bioavailability and improved user compliance.
Objectives: To compare the contraceptive effectiveness, cycle control, compliance (adherence), and safety of the contraceptive patch or the vaginal ring versus combination oral contraceptives (COCs).
Search methods: Through February 2013, we searched MEDLINE, POPLINE, CENTRAL, LILACS, ClinicalTrials.gov, and ICTRP for trials of the contraceptive patch or the vaginal ring. Earlier searches also included EMBASE. For the initial review, we contacted known researchers and manufacturers to identify other trials.
Selection criteria: We considered randomized controlled trials comparing a transdermal contraceptive patch or a contraceptive vaginal ring with a COC.
Data collection and analysis: Data were abstracted by two authors and entered into RevMan. For dichotomous variables, the Peto odds ratio (OR) with 95% confidence intervals (CI) was calculated. For continuous variables, the mean difference was computed. We also assessed the quality of evidence for this review.
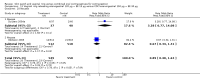
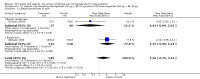
Main results: We found 18 trials that met our inclusion criteria. Of six patch studies, five examined the marketed patch containing norelgestromin plus ethinyl estradiol (EE); one studied a patch in development that contains levonorgestrel (LNG) plus EE. Of 12 vaginal ring trials, 11 examined the same marketing ring containing etonogestrel plus EE; one studied a ring being developed that contains nesterone plus EE.Contraceptive effectiveness was not significantly different for the patch or ring versus the comparison COC. Compliance data were limited. Patch users showed better compliance than COC users in three trials. For the norelgestromin plus EE patch, ORs were 2.05 (95% CI 1.83 to 2.29) and 2.76 (95% CI 2.35 to 3.24). In the levonorgestrel plus EE patch report, patch users were less likely to have missed days of therapy (OR 0.36; 95% CI 0.25 to 0.51). Of four vaginal ring trials, one found ring users had more noncompliance (OR 3.99; 95% CI 1.87 to 8.52), while another showed more compliance with the regimen (OR 1.67; 95% CI 1.04 to 2.68).More patch users discontinued early than COC users. ORs from two meta-analyses were 1.59 (95% CI 1.26 to 2.00) and 1.56 (95% CI 1.18 to 2.06) and another trial showed OR 2.57 (95% CI 0.99 to 6.64). Patch users also had more discontinuation due to adverse events than COC users. Users of the norelgestromin-containing patch reported more breast discomfort, dysmenorrhea, nausea, and vomiting. In the levonorgestrel-containing patch trial, patch users reported less vomiting, headaches, and fatigue.Of 11 ring trials with discontinuation data, two showed the ring group discontinued less than the COC group: OR 0.32 (95% CI 0.16 to 0.66) and OR 0.52 (95% CI 0.31 to 0.88). Ring users were less likely to discontinue due to adverse events in one study (OR 0.32; 95% CI 0.15 to 0.70). Compared to the COC users, ring users had more vaginitis and leukorrhea but less vaginal dryness. Ring users also reported less nausea, acne, irritability, depression, and emotional lability than COC users.For cycle control, only one trial study showed a significant difference. Women in the patch group were less likely to have breakthrough bleeding and spotting. Seven ring studies had bleeding data; four trials showed the ring group generally had better cycle control than the COC group.
Authors' conclusions: Effectiveness was not significantly different for the methods compared. Pregnancy data were available from half of the patch trials but two-thirds of ring trials. The patch could lead to more discontinuation than the COC. The patch group had better compliance than the COC group. Compliance data came from half of the patch studies and one-third of the ring trials. Patch users had more side effects than the COC group. Ring users generally had fewer adverse events than COC users but more vaginal irritation and discharge.The quality of the evidence for this review was considered low for the patch and moderate for the ring. The main reasons for downgrading were lack of information on the randomization sequence generation or allocation concealment, the outcome assessment methods, high losses to follow up, and exclusions after randomization.
Conflict of interest statement
D Grimes has consulted with Bayer Healthcare Pharmaceuticals and Merck & Co., Inc.
Figures












































































































































































Update of
-
Skin patch and vaginal ring versus combined oral contraceptives for contraception.Cochrane Database Syst Rev. 2010 Mar 17;(3):CD003552. doi: 10.1002/14651858.CD003552.pub3. Cochrane Database Syst Rev. 2010. Update in: Cochrane Database Syst Rev. 2013 Apr 30;(4):CD003552. doi: 10.1002/14651858.CD003552.pub4. PMID: 20238323 Updated.
References
References to studies included in this review
Ahrendt 2006 {published data only}
-
- Ahrendt H‐J, Nisand I, Bastianelli C, Gómez AM, Gemzell‐Danielsson K, Urdl W, et al. Efficacy, acceptability and tolerability of the combined contraceptive ring, NuvaRing, compared with an oral contraceptive containing 3 µg of ethinyl estradiol and 3 mg of drospirenone. Contraception 2006;74(6):451‐7. - PubMed
-
- Milsom I, Lete I, Bjertnaes A, Rokstad K, Lindh I, Gruber CJ, et al. Effects on cycle control and bodyweight of the combined contraceptive ring, NuvaRing, versus an oral contraceptive containing 30 µg ethinyl estradiol and 3 mg drospirenone. Human Reproduction 2006;21(9):2304‐11. - PubMed
Audet 2001 {published data only}
-
- Audet MC, Moreau M, Koltun WD, Waldbaum AS, Shangold G, Fisher AC, et al. Evaluation of contraceptive efficacy and cycle control of a transdermal contraceptive patch vs an oral contraceptive: a randomized controlled trial. Journal of the American Medical Association 2001;285(18):2347‐54. - PubMed
Boonyarangkul 2007 {published data only}
-
- Boonyarangkul A, Taneepanichskul S. Comparison of cycle control and side effects between transdermal contraceptive patch and an oral contraceptive in women older than 35 years. Journal of the Medical Association of Thailand 2007;90(9):1715‐9. - PubMed
Dittrich 2002 {published data only}
-
- Dittrich R, Parker L, Rosen JB, Shangold G, Creasy GW, Fisher AC. Transdermal contraception: evaluation of three transdermal norelgestromin/ethinyl estradiol doses in a randomized, multicenter, dose‐response study. American Journal of Obstetrics and Gynecology 2002;186(1):15‐20. - PubMed
Duijkers 2004a {published and unpublished data}
-
- Duijkers I, Killick S, Bigrigg A, Dieben TO. A comparative study on the effects of a contraceptive vaginal ring NuvaRing and an oral contraceptive on carbohydrate metabolism and adrenal and thyroid function. European Journal of Contraception and Reproductive Health Care 2004;9(3):131‐40. - PubMed
Elkind‐Hirsch 2007 {published data only}
-
- Elkind‐Hirsch KE, Darensbourg C, Ogden B, Ogden LF, Hindelang P. Contraceptive vaginal ring use for women has less adverse metabolic effects than an oral contraceptive. Contraception 2007;76(5):348‐56. - PubMed
Gilliam 2010 {published data only}
-
- Gilliam M L, Neustadt A, Kozloski M, Mistretta S, Tilmon S, Godfrey E. Adherence and acceptability of the contraceptive ring compared with the pill among students: a randomized controlled trial. Obstetrics and Gynecology 2010;115(3):503‐10. - PubMed
-
- Hughey A B, Neustadt A B, Mistretta S Q, Tilmon S J, Gilliam M L. Daily context matters: predictors of missed oral contraceptive pills among college and graduate students. American Journal of Obstetrics and Gynecology 2010;203(4):323 e1‐7. - PubMed
Guida 2005 {published data only}
-
- Guida M, Spiezio Sardo A, Bramante S, Sparice S, Acunzo G, Tommaselli GA, et al. Effects of two types of hormonal contraception‐‐oral versus intravaginal‐‐on the sexual life of women and their partners. Human Reproduction 2005;20(4):1100‐6. - PubMed
Kaunitz 2012 {published data only (unpublished sought but not used)}
-
- Kaunitz A, Archer DF, Foegh M. Increased compliance with a low‐dose combination contraceptive patch (AG200‐15) compared with a low‐dose combination oral contraceptive (COC) in a Phase 3 clinical trial (abstract). Contraception 2012;86(2):178.
-
- Kaunitz A, Foegh M, Mishell D. Increased compliance independent of patient demographics with a low‐dose combination contraceptive patch (AG200‐15) compared with a low‐dose combination oral contraceptive in a Phase 3 clinical trial (abstract). Contraception 2012;86(3):314.
-
- Kaunitz AM, Mishell DR, Foegh M. Comparative Phase 3 study of AG200‐15, a low‐dose estrogen and levonorgestrel contraceptive patch. http://www.agiletherapeutics.com/Clinical_support.html (accessed 26 Sep 2012).
Kluft 2008 {published data only}
-
- Kluft C, Mayer G, Helmerhorst FM, Hall H, Creasy G. Comparison of the effects of a contraceptive patch and oral contraceptives on coagulation parameters [abstract no. FC2.30.04]. International Journal of Gynaecology and Obstetrics 2000;70(Suppl 2):B77.
-
- Kluft C, Meijer P, LaGuardia KD, Fisher AC. Comparison of a transdermal contraceptive patch vs. oral contraceptives on hemostasis variables. Contraception 2008;77(2):77‐83. - PubMed
Mohamed 2011 {published data only (unpublished sought but not used)}
-
- Mohamed A M, El‐Sherbiny W S, Mostafa W A. Combined contraceptive ring versus combined oral contraceptive (30‐mug ethinylestradiol and 3‐mg drospirenone). International Journal of Gynaecology and Obstetrics 2011;114(2):145‐8. - PubMed
Oddsson 2005 {published data only}
-
- Oddsson K, Leifels‐Fischer B, Wiel‐Masson D, Melo NR, Benedetto C, Verhoeven CHJ, et al. Superior cycle control with a contraceptive vaginal ring compared with an oral contraceptive containing 30 µg ethinylestradiol and 150 µg levonorgestrel: a randomized trial. Human Reproduction 2005;20(2):557‐62. - PubMed
-
- Oddsson K, Leifels‐Fischer B, Melo NR, Wiel‐Masson D, Benedetto C, Verhoeven CHJ, et al. Efficacy and safety of a contraceptive vaginal ring (NuvaRing) compared with a combined oral contraceptive: a 1‐year randomized trial. Contraception 2005;71(3):176‐82. - PubMed
Rad 2006 {published data only}
-
- Rad M, Kluft C, Menard J, Burggraaf J, Kam ML, Meijer P, et al. Comparative effects of a contraceptive vaginal ring delivering a nonandrogenic progestin and continuous ethinyl estradiol and a combined oral contraceptive containing levonorgestrel on hemostasis variables. American Journal of Obstetrics and Gynecology 2006;195(1):72‐7. - PubMed
-
- Sitruk‐Ware RL, Menard J, Rad M, Burggraaf J, Kam ML, Tokay BA, et al. Comparison of the impact of vaginal and oral administration of combined hormonal contraceptives on hepatic proteins sensitive to estrogen. Contraception 2007;75(6):430‐7. - PubMed
Sabatini 2006 {published data only}
-
- Sabatini R, Cagiano R. Comparison profiles of cycle control, side effects and sexual satisfaction of three hormonal contraceptives. Contraception 2006;74(3):220‐3. - PubMed
Stewart 2007 {published data only}
Urdl 2005 {published data only}
-
- Hedon B, Helmerhorst FM, Cronje HS, Shangold G, Fisher A, Creasy G. Comparison of efficacy, cycle control, compliance and safety in users of a contraceptive patch vs an oral contraceptive. International Journal of Gynaecology and Obstetrics 2000;70(Suppl 2):B78.
-
- Urdl W, Apter D, Alperstein A, Koll P, Schönian S, Bringer J, et al. Contraceptive efficacy, compliance and beyond: factors related to satisfaction with once‐weekly transdermal compared with oral contraception. European Journal of Obstetrics & Gynecology and Reproductive Biology 2005;121(2):202‐10. - PubMed
Veres 2004 {published data only}
-
- Veres S, Miller L, Burington B. A comparison between the vaginal ring and oral contraceptives. Obstetrics and Gynecology 2004;104(3):555‐63. - PubMed
Westhoff 2005 {published data only}
-
- O'Connell KJ, Osborne LM, Westhoff C. Measured and reported weight change for women using a vaginal contraceptive ring vs. a low‐dose oral contraceptive. Contraception 2005;72(5):323‐7. - PubMed
-
- Schafer JE, Osborne LM, Davis AR, Westhoff C. Acceptability and satisfaction using Quick Start with the contraceptive vaginal ring versus an oral contraceptive. Contraception 2006;73(5):488‐92. - PubMed
-
- Westhoff C, Osborne LM, Schafer JE, Morroni C. Bleeding patterns after immediate initiation of an oral compared with a vaginal hormonal contraceptive. Obstetrics and Gynecology 2005;106(1):89‐96. - PubMed
References to studies excluded from this review
Archer 2002 {published data only}
-
- Archer D, Bigrigg A, Smallwood G, Shangold G, Creasy G. An integrated assessment of patient compliance with a weekly contraceptive patch (ORTHO EVRA / EVRA). Fertility and Sterility 2001; Vol. 76 (3 Suppl 1):20. - PubMed
-
- Archer DF, Bigrigg A, Smallwood GH, Shangold GA, Creasy GW, Fisher AC. Assessment of compliance with a weekly contraceptive patch (Ortho Evra™/Evra™) among North American women. Fertility and Sterility 2002;77:27‐31. - PubMed
Archer 2004 {published data only}
-
- Archer DF, Cullins V, Creasy GW, Fisher AC. The impact of improved compliance with a weekly contraceptive transdermal system (Ortho Evra) on contraceptive efficacy. Contraception 2004;69:189‐95. - PubMed
Bednarek 2008 {published data only}
-
- Bednarek PH, Nichols MD, Carlson N, Edelman AB, Creinin MD, Truitt S, et al. Effect of "observed start" vs. traditional "Sunday start" on hormonal contraceptive continuation rates after medical abortion. Contraception 2008;78(1):26‐30. - PubMed
Bjarnadottir 2002 {published data only}
-
- Bjarnadottir RI, Tuppurainen M, Killick SR. Comparison of cycle control with a combined contraceptive vaginal ring and oral levonorgestrel/ethinyl estradiol. American Journal of Obstetrics and Gynecology 2002;186:389‐95. - PubMed
Bustillos‐Alamilla 2010 {published data only}
-
- Bustillos‐Alamilla E, Zepeda‐Zaragoza J, Hernandez‐Ruiz M A, Briones‐Landa C H. Combined hormonal contraception in cycles artificially extended [Anticoncepción con hormonales combinados en ciclos extendidos artificialmente]. Ginecología de Obstetricia de México 2010;78(1):37‐45. - PubMed
Creasy 2000 {published data only}
-
- Creasy G, Hall N, Shangold G. Patient adherence with the contraceptive patch dosing schedule versus oral contraceptives. Obstetrics and Gynecology 2000; Vol. 95:S60.
Duijkers 2004b {published data only}
-
- Duijkers IJM, Klipping C, Verhoeven CHJ, Dieben TOM. Ovarian function with the contraceptive vaginal ring or an oral contraceptive: a randomized study. Human Reproduction 2004;19:2668‐73. - PubMed
-
- Duijkers IJM, Verhoeven CHJ, Dieben TOM, Klipping C. Follicular growth during contraceptive pill or vaginal ring treatment depends on the day of ovulation in the pretreatment cycle. Human Reproduction 2004;19:2674‐9. - PubMed
Guazzelli 2011 {published data only}
-
- Guazzelli CA, Barreiros FA, Barbosa R, Torloni MR, Barbieri M. Extended regimens of the contraceptive vaginal ring versus hormonal oral contraceptives: effects on lipid metabolism. Contraception 2011. - PubMed
Mulders 2001 {published data only}
-
- Mulders TM, Dieben TO. Use of the novel combined contraceptive vaginal ring NuvaRing for ovulation inhibition. Fertility and Sterility 2001;75:865‐70. - PubMed
Pierson 2000 {published data only}
-
- Pierson RA, Hess AR, Archer DF, Parsons K, Shangold G, Fisher A, et al. Effects of a contraceptive patch and 3 oral contraceptives on follicular development following incorrect dosing [abstract no. FC2.30.08]. International Journal of Gynaecology and Obstetrics 2000;70:78.
Pierson 2003 {published data only}
-
- Pierson RA, Archer DF, Moreau M, Shangold GA, Fisher AC, Creasy GW. Ortho Evra/Evra versus oral contraceptives: follicular development and ovulation in normal cycles and after an intentional dosing error. Contraception 2003;80:34‐42. - PubMed
Roumen 2006 {published data only}
-
- Roumen FJ, Dieben TO. Comparison of uterine concentrations of ethinyl estradiol and etonogestrel after use of a contraceptive vaginal ring and an oral contraceptive. Fertility and Sterility 2006;85:57‐62. - PubMed
Sibai 2002 {published data only}
-
- Sibai BM, Odlind V, Meador ML, Shangold GA, Fisher AC, Creasy GW. A comparative and pooled analysis of the safety and tolerability of the contraceptive patch (Ortho Evra™/Evra™). Fertility and Sterility 2002;77:19‐26. - PubMed
Smallwood 2001 {published data only}
-
- Smallwood GH, Meador ML, Lenihan JP, Shangold GA, Fisher AC, Creasy GW. Efficacy and safety of a transdermal contraceptive system. Obstetrics and Gynecology 2001;98(5 Pt 1):799‐805. - PubMed
Timmer 2000 {published data only}
-
- Timmer CJ, Mulders TM. Pharmacokinetics of etonogestrel and ethinylestradiol released from a combined contraceptive vaginal ring. Clinical Pharmacokinetics 2000;39:233‐42. - PubMed
Van den Heuvel 2005 {published data only}
-
- Heuvel MW, Bragt AJ, Alnabawy AK, Kaptein MC. Comparison of ethinylestradiol pharmacokinetics in three hormonal contraceptive formulations: the vaginal ring, the transdermal patch and an oral contraceptive. Contraception 2005;72:168‐74. - PubMed
White 2005 {published data only}
-
- White T, Jain JK, Stanczyk FZ. Effect of oral versus transdermal steroidal contraceptives on androgenic markers. American Journal of Obstetrics and Gynecology 2005;192:2055‐9. - PubMed
-
- White T, Özel B, Jain JK, Stanczyk FZ. Effects of transdermal and oral contraceptives on estrogen‐sensitive hepatic proteins. Contraception 2006;74:293‐6. - PubMed
Zacur 2002 {published data only}
-
- Zacur HA, Hedon B, Mansour D, Shangold GA, Fisher AC, Creasy GW. Integrated summary of Ortho Evra™/Evra™ contraceptive patch adhesion in varied climates and conditions. Fertility and Sterility 2002;77:32‐5. - PubMed
Zieman 2002 {published data only}
-
- Zieman M, Guillebaud J, Weisberg E, Shangold GA, Fisher AC, Creasy GW. Contraceptive efficacy and cycle control with the Ortho Evra™/Evra™ transdermal system. Fertility and Sterility 2002;77:13‐8. - PubMed
References to studies awaiting assessment
Agile 2011 {published data only (unpublished sought but not used)}
-
- Agile Therapeutics. Transdermal contraceptive delivery system (TCDS) of levonorgestrel and ethinyl estradiol (ATI‐CL13). http://clinicaltrials.gov/ct2/show/NCT01236768 (accessed 27 Sep 2012). [NCT01236768]
Bayer 2012b {published and unpublished data}
-
- Bayer Healthcare AG. US cycle control and blood pressure study. http://clinicaltrials.gov/ct2/show/NCT00920985 (accessed 27 Sep 2012). [NCT00920985]
Weisberg 2012 {published data only}
-
- Weisberg E. Comparative 12 month study of menstrually‐signalled use of a combined contraceptive pill versus a combined contraceptive vaginal ring. http://www.anzctr.org.au/ACTRN12609000391279.aspx (accessed 26 Jul 2012).
Additional references
Als‐Nielsen 2003
-
- Als‐Nielsen B, Chen W, Gluud C, Kjaergard LL. Association of funding and conclusions in randomized drug trials: a reflection of treatment effect or adverse events?. Journal of the American Medical Association 2003;290:921‐8. - PubMed
Bayer 2012a
-
- Bayer Healthcare AG. Inhibition of ovulation and pharmacokinetics of transdermal ethinylestradiol (EE) and gestodene (GSD). http://clinicaltrials.gov/ct2/show/NCT00915915 (accessed 01 Oct 2012). [DOI: ]
Brache 2013
-
- Brache V, Payan LJ, Faundes A. Current status of contraceptive vaginal rings. Contraception 2013;87(3):264‐72. - PubMed
CONSORT 2009
-
- CONSORT group. CONSORT: Transparent reporting of trials. http://www.consort‐statement.org/ (accessed 15 Jul 2009).
Deeks 2001
-
- Deeks JJ, Altman DG, Bradburn MJ. Statistical methods for examining heterogeneity and combining results from several studies in meta‐analysis. In: Egger M, Smith GD, Altman DG editor(s). Systematic Reviews in Health Care: Meta‐analysis in Context. 2nd Edition. London: BMJ Publishing Group, 2001:285‐312.
FDA 2012
-
- US Food, Drug Administration. Ortho Evra (norelgestromin/ethinyl estradiol) information. http://www.fda.gov/Drugs/DrugSafety/PostmarketDrugSafetyInformationforPa... (accessed 08 Nov 2012).
Grimes 2009
-
- Grimes DA. Forgettable contraception. Contraception 2009;80:497‐9. - PubMed
Haag 1998
-
- Haag U. Technologies for automating randomized treatment assignment in clinical trials. Drug Information Journal 1998;32:7‐11.
Heit 2005
-
- Heit JA, Kobbervig CE, James AH, Petterson TM, Bailey KR, Melton LJ III. Trends in the incidence of venous thromboembolism during pregnancy or postpartum: a 30‐year population‐based study. Annals of Internal Medicine 2005;143:697‐706. - PubMed
Henzl 2000
-
- Henzl MR, Edwards JA. Pharmacology of progestins: 17alpha‐hydroxyprogesterone derivatives and progestins of the first and second generation. In: Sitruk‐Ware R, Mishell DR editor(s). Progestins and Antiprogestins in Clinical Practice. New York (NY): Marcel Dekker, 2000:101‐32.
Higgins 2011
-
- Higgins JPT, Green S, editors. Cochrane Handbook for Systematic Reviews of Interventions 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org. John Wiley & Sons, Ltd, (accessed 24 October 2012).
Hou 2010
-
- Hou MY, Hurwitz S, Kavanagh E, Fortin J, Goldberg AB. Using daily text‐message reminders to improve adherence with oral contraceptives. Obstetrics and Gynecology 2010;116(3):633‐40. [NCT00733707] - PubMed
Jensen 2013
Langston 2010
-
- Langston AM, Rosario L, Westhoff CL. Structured contraceptive counseling‐‐a randomized controlled trial. Patient Education and Counseling. 2010/09/28 2010; Vol. 81, issue 3:362‐7. - PubMed
Lete 2008
-
- Lete I, Doval JL, Perez‐Campos E, Lertxundi R, Correa M, Viuda E, et al. Self‐described impact of noncompliance among users of a combined hormonal contraceptive method. Contraception. 2008/03/18 2008; Vol. 77, issue 4:276‐82. - PubMed
Lexchin 2003
Moher 2001
-
- Moher D, Schulz KF, Altman DG. The CONSORT statement: revised recommendations for improving the quality of reports of parallel‐group randomized trials. Lancet 2001;357:1191‐4. - PubMed
NICHD 2012
-
- Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD). A multi‐center, open‐label, randomized, parallel group study to evaluate pharmacokinetic profile, effects on the mechanisms of contraceptive efficacy and safety of two progestin‐only patches containing different doses of levonorgestrel (LNG) (CCN009). http://clinicaltrials.gov/ct2/show/NCT01166412 (accessed 23 Sep 2012). [DOI: ]
Pop Council 2012
-
- Population Council. Licensing Opportunities. http://www.popcouncil.org/what/licensing.asp#/jQueryUITabs1‐2 (accessed 12 Nov 2012).
Potter 1996
-
- Potter L, Oakley D, Leon‐Wong E, Canamar R. Measuring compliance among oral contraceptive users. Family Planning Perspectives 1996;28:154‐8. - PubMed
Raymond 2012
-
- Raymond EG, Burke AE, Espey E. Combined hormonal contraceptives and venous thromboembolism: putting the risks into perspective. Obstetrics and Gynecology 2012; Vol. 119, issue 5:1039‐44. - PubMed
Rosenberg 1998
-
- Rosenberg MJ, Waugh MS, Burnhill MS. Compliance, counseling and satisfaction with oral contraceptives: a prospective evaluation. Family Planning Perspectives 1998;30:89‐92,104. - PubMed
Schulz 1995
-
- Schulz KF, Chalmers I, Hayes RJ, Altman DG. Dimensions of methodological quality associated with estimates of treatment effects in controlled trials. Journal of the American Medical Association 1995;273:408‐12. - PubMed
Schulz 2002a
-
- Schulz KF, Grimes DA. Allocation concealment in randomised trials: defending against deciphering. Lancet 2002;359:614‐8. - PubMed
Sitruk‐Ware 2013
Stanczyk 2011
-
- Stanczyk FZ, Rubin A, Flood L, Foegh M. Pharmacokinetics, tolerability and cycle control of three transdermal contraceptive delivery systems containing different doses of ethinylestradiol and levonorgestrel. Hormone Molecular Biology and Clinical Investigation 2011;6(2):231‐40. - PubMed
Strauss 2005
-
- Strauss SE, Richardson WS, Glasziou P, Haynes RB. Evidence‐based Medicine: How to Practice and Teach EBM. Third Edition. New York: Churchill Livingstone, 2005.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

