Oral evening primrose oil and borage oil for eczema
- PMID: 23633319
- PMCID: PMC8105655
- DOI: 10.1002/14651858.CD004416.pub2
Oral evening primrose oil and borage oil for eczema
Abstract
Background: Eczema is a chronic inflammatory skin condition, which usually develops in early childhood. Many children outgrow this disorder as they reach secondary school age, and although It may improve with age, there is no cure. Constant itch makes life uncomfortable for those with this condition, no matter what age they are, so it may have a significant effect on a person's quality of life. Its prevalence seems to be increasing as populations move from rural locations to cities. Some people, who do not see an adequate improvement or fear side-effects of conventional medical products, try complementary alternatives to conventional treatment. This is a review of evening primrose oil (EPO) and borage oil (BO) taken orally (by mouth); these have been thought to be beneficial because of their gamma-linolenic acid content.
Objectives: To assess the effects of oral evening primrose oil or borage oil for treating the symptoms of atopic eczema.
Search methods: We searched the following databases up to August 2012: Cochrane Skin Group Specialised Register, CENTRAL in The Cochrane Library, MEDLINE (from 1946), EMBASE (from 1974), AMED (from 1985), and LILACS (from 1982). We also searched online trials registers and checked the bibliographies of included studies for further references to relevant trials. We corresponded with trial investigators and pharmaceutical companies to try to identify unpublished and ongoing trials. We performed a separate search for adverse effects of evening primrose oil and borage oil in November 2011.
Selection criteria: All randomised controlled, parallel, or cross-over trials investigating oral intake of evening primrose oil or borage oil for eczema.
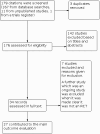
Data collection and analysis: Two review authors independently applied eligibility criteria, assessed risk of bias, and extracted data. We pooled dichotomous outcomes using risk ratios (RR), and continuous outcomes using the mean difference (MD). Where possible, we pooled study results using random-effects meta-analysis and tested statistical heterogeneity using both the Chi(²) test and the I(²) statistic test. We presented results using forest plots with 95% confidence intervals (CI).
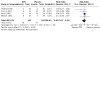
Main results: A total of 27 studies (1596 participants) met the inclusion criteria: 19 studies assessed evening primrose oil, and 8 studies assessed borage oil. For EPO, a meta-analysis of results from 7 studies showed that EPO failed to significantly increase improvement in global eczema symptoms as reported by participants on a visual analogue scale of 0 to 100 (MD -2.22, 95% CI -10.48 to 6.04, 176 participants, 7 trials) and a visual analogue scale of 0 to 100 for medical doctors (MD -3.26, 95% CI -6.96 to 0.45, 289 participants, 8 trials) compared to the placebo group.Treatment with BO also failed to significantly improve global eczema symptoms compared to placebo treatment as reported by both participants and medical doctors, although we could not conduct a meta-analysis as studies reported results in different ways. With regard to the risk of bias, the majority of studies were of low risk of bias; we judged 67% of the included studies as having low risk of bias for random sequence generation; 44%, for allocation concealment; 59%, for blinding; and 37%, for other biases.
Implications for practice: Oral borage oil and evening primrose oil lack effect on eczema; improvement was similar to respective placebos used in trials. Oral BO and EPO are not effective treatments for eczema.In these studies, along with the placebos, EPO and BO have the same, fairly common, mild, transient adverse effects, which are mainly gastrointestinal.The short-term studies included here do not examine possible adverse effects of long-term use of EPO or BO. A case report warned that if EPO is taken for a prolonged period of time (more than one year), there is a potential risk of inflammation, thrombosis, and immunosuppression; another study found that EPO may increase bleeding for people on Coumadin® (warfarin) medication.
Implications for research: Noting that the confidence intervals between active and placebo treatment are narrow, to exclude the possibility of any clinically useful difference, we concluded that further studies on EPO or BO for eczema would be hard to justify.This review does not provide information about long-term use of these products.
Conflict of interest statement
Joel Bamford's research was funded with a grant from the Miller‐Dwan Foundation and repaid by the Efamol Pharmaceutical company, which supplied medication, placebo, and blood testing. Joel Bamford was neither involved in the selection nor data extraction of the Bamford 1985 included study for which he is a first author. Instead, the third review author (AM) took up these roles in the case of this included study.
Figures



























Update of
References
References to studies included in this review
Bahmer 1992 {published data only (unpublished sought but not used)}
-
- Bahmer FA, Schafer J. Treatment of Atopic Dermatitis with Borage Seed Oil (Glandol) - a Time Series Analysis Study [Die Behandlung der atopischen Dermatitis mit Borretschsamen-Ol (Glandol)--eine zeitreihenanalytische Studie]. Kinderarztliche Praxis 1992;60(7):199-202. [MEDLINE: ] - PubMed
Bamford 1985 {published and unpublished data}
-
- Bamford JT, Gibson RW, Renier CM. Atopic eczema unresponsive to evening primrose oil (linoleic and gammma-linolenic acids). Journal of the American Academy of Dermatology 1985;13(6):959-65. [PMID: ] - PubMed
Berth‐Jones 1993 {published data only}
-
- Berth-Jones J, Graham-Brown RA. Placebo-controlled trial of essential fatty acid supplementation in atopic dermatitis. Lancet 1993;341(8860):1557-60. [MEDLINE: ] - PubMed
Biagi 1994 {published data only (unpublished sought but not used)}
-
- Biagi PL, Bordoni A, Hrelia S, Celadon M, Ricci GP, Cannella V, et al. The effect of gamma-linolenic acid on clinical status, red cell fatty acid composition and membrane microviscosity in infants with atopic dermatitis. Drugs Under Experimental & Clinical Research 1994;20(2):77-84. [MEDLINE: ] - PubMed
Borrek 1997 {published data only}
-
- Borrek S, Hildebrandt A, Forster J. Gamma-linolenic-acid-rich borage seed oil capsules in children with atopic dermatitis. A placebo-controlled double-blind study [Gammalinolensaure-reiche Borretschsamenol-Kapseln bei Kindern mit Atopischer Dermatitis. Eine placebo-kontrollierte Doppelblind-Studie]. Klinische Padiatrie 1997;209(3):100-4. - PubMed
Buslau 1996 {published data only}
-
- Buslau M, Thaci D. Atopic dermatitis: Borage oil for systemic therapy [Atopische Dermatitis: Borretschol zur systemischen Therapie]. Zeitschrift fur Dermatologie 1996;182(3):131-6.
Don 2003 {published data only (unpublished sought but not used)}
-
- Don M, Melli P, Braida F, Comici A, Barbi E, Spano C, et al. Efficacy of essential fatty acids in the treatment of atopic dermatitis and correlations of their changes with clinical response [Efficacia degli acidi grassi essenziali nel trattamento della dermatite atopica e correlazione de; loro cambiamenti con la risposta clinica]. Italian Journal of Pediatrics 2003;29(6):427-32.
Finlay 2001 {unpublished data only}
-
- Finlay AY, Marks R, Wilson AM, Huntley C. Epogam in Children with Atopic Eczema. Pharmacia (Formerly Searle Division of Monsanto) 2001:1-473.
France 1988 {unpublished data only}
-
- France et al. Epogam in Severe Atopic Eczema. Pharmacia 2001(18 October). [ISN 880002/3/4/24/25/128/130/890141]
Fraser 2001 {unpublished data only}
-
- Fraser. Efamol in Atopic Eczema. Pharmacia 2001 (22 October). [ISSN 86004/5/6/7]
Harper 1990 {unpublished data only}
-
- Harper J statician authors: Jaderberg M and Wilson A. Efamol in Moderate to Severe Atopic Eczema. Pharmacia 2001. [SN 890136/7]
Heddle 1990 {unpublished data only}
-
- Heddle RJ, Forsyth K, Gordon LA, TAO BK, Gibson RA. Oral Evening Primrose Oil (EPO) in Atopic Eczema. Pharmacia 2001. [ISN 910033]
Hederos 1996 {published data only}
Henz 1999 {published data only}
-
- Henz BM, Jablonska S, de Kerkhof PC, Stingl G, Blaszczyk M, Vandervalk PG, et al. Double-blind, multicenter analysis of the efficacy of borage oil in patients with atopic eczema. British Journal of Dermatology 1999;140(4):685-8. [MEDLINE: ] - PubMed
Kenicer 2001 {unpublished data only}
-
- Kenicer K, Keczkes K, Green C, George S. Evening Primrose Oil in the Treatment of Atopic Dermatitis: a Placebo-controlled Study. Pharmacia Company 2001. [ISN: 900210/910098]
Kiehl 1994 {published data only}
-
- Kiehl R, Ionescu G, Manuel P, Stern LP, Peters G, Niemann A, et al. Clinical, Immuno- and Lipid Modulating Effects of Unsaturated Fatty Acids in Patients with Atopic Eczema [Klinische, immun- und lipidmodulatorische effekte einer behandlung mit ungesattigten fettsauren bei atopischer dermatitis]. H+G Zeitschrift fur Hautkrankheiten 1994;69(1):42-8.
Kovar 1992 {unpublished data only}
-
- Kovar IZ, Wright S. An assessment of efamol evening primrose oil in moderate to severe atopic eczema in young children. Pharmacia Corporation 1992. [ISN 900158]
Lovell 1981 {published and unpublished data}
-
- Lovell CR, Burton JL, Horrobin DF. Treatment of atopic eczema with evening primrose oil. Lancet 1981;1(8214):278. [PMID: ] - PubMed
MacKie Adult 1990 {unpublished data only}
-
- MacKie R. A Clinical and Mechanistic Assessment of Eamol Evening Primrose Oil in Atopic Eczema (Adults). Pharmacia 2001. [ISN 890054]
MacKie Child 1990 {published data only}
-
- MacKie R. A clinical and mechanistic assessment of efamol evening primrose oil in atopic eczema in Children. Pharmacia 2001. [ISN 890067]
MacLeod 2001 {unpublished data only}
-
- MacLeod A, Misch KJ. A Comparative Assessment of Efamol Evening Primrose Oil and Efamol Marine in Severe Atopic Eczema. Pharmacia 2001. [ISN 89100]
Schalin‐Karrila 1987 {published data only}
-
- Schalin-Karrila M, Mattila L, Jansen CT, Uotila P. Evening primrose oil in the treatment of atopic eczema: effect on clinical status, plasma phospholipid fatty acids and circulating blood prostaglandins. British Journal of Dermatology 1987;117(1):11-9. [PMID: ] - PubMed
Senapati 2008 {published data only}
-
- Senapati S, Banerjee S, Gangopadhyay DN. Evening primrose oil is effective in atopic dermatitis: A randomized placebo-controlled trial. Indian Journal of Dermatology, Venereology and Leprology 2008;74(5):447-52. [PMID: ] - PubMed
Takwale 2003 {published data only}
Valsecchi 2006 {published data only}
-
- Valsecchi R, Di Landro A, Pansera B, Reseghetti A. Gamma-lenolenic acid in the treatment of atopic dermatitis. Journal of European Academy of Dermatology & Venereology 2006 July;7(1):77-9.
Wishart 1992 {unpublished data only}
-
- Wishart J, Macdonald K, Oakley A. A Multi-center Assessment of Efamol Evening Primrose Oil in Severe Atopic Eczema. Pharmacia October 2001. [ISN 900196/197/198]
Wright 1982 {published data only (unpublished sought but not used)}
-
- Wright S, Burton JL. Oral evening-primrose-seed oil improves atopic eczema. Lancet 1982;2(8308):1120-2. [6128449] - PubMed
References to studies excluded from this review
Biagi 1988 {published data only (unpublished sought but not used)}
-
- Biagi PL, Bordoni A, Masi M, Ricci G, Fanelli C, Patrizi A, et al. A long-term study on the use of evening primrose oil (Efamol) in atopic children. Drugs Under Experimental & Clinical Research 1988;14(4):285-90. [MEDLINE: ] - PubMed
Bordoni 1988 {published data only}
-
- Bordoni A, Biagi PL, Masi M, Ricci G, Fanelli C, Patrizi A, et al. Evening primrose oil (Efamol) in the treatment of children with atopic eczema. Drugs Under Experimental & Clinical Research 1988;14(4):291-7. [PMID: ] - PubMed
Coskery 1988 {published data only (unpublished sought but not used)}
-
- Coskery G, Cowley N, Allen R. The Effect of Dietary Supplementation with Evening Primrose Oil on Skin Surface Texture in Atopic Dermatitis, A quantitative method of assessment. [Abstract 216]. The 18th Annual Meeting of the European Society for Dermatologic Research 19-22 June 1988, Munich, Germany. Journal of Investigative Dermatology 1988;91(4):413.
Courage 1991 {published data only}
-
- Courage B, Nissen HP, Wehrmann W, Biltz H. Influence of polyunsaturated Fatty Acids on the Plasma Catecholamines of Patients with Atopic Eczema [Veranderungen des Katecholaminspiegels bei Neurodermatitis - Beeinflussung durch ungesattigte Fettsauren]. H+G Zeitschrift fur Hautkrankheiten 1991;66(6):509-10. [EMBASE: 1991249008]
Guenther 1987 {published data only (unpublished sought but not used)}
-
- Guenther L, Wexler D. Efamol in the treatment of atopic dermatitis. Journal of the American Academy of Dermatology 1987;17(5 Pt 1):860. - PubMed
Melnick 1995 {published data only}
-
- Melnik BC, Bahmer FA. Treatment of Atopic Dermatitis with Glandol and Epoleum - A Comparative Study [Die behandlung des atopischen ekzems mit glandol und epoleum - eine vergleichende studie]. Aktuelle Dermatologie 1995;21(7):215-9.
NCT00878670 {unpublished data only}
-
- NCT00878670. Investigation of Efficacy and Safety of EPOGAM. clinicaltrials.gov/ct2/show/NCT00878670 (accessed 16 December 2012).
Swapan 2008 {published data only}
-
- Swapan S, Banerjee S, Gangopadhyay DN. Evening primrose oil is effective in atopic dermatitis: A randomized placebo-controlled trial. Indian Journal of Dermatology, Venereology & Leprology 2008;74(5):447-52. [PMID: ] - PubMed
Additional references
Al‐Khamees 2011
Augustin 1999
-
- Augustin M, Zschocke I, Buhrke U. Attitudes and prior experience with respect to alternative medicine among dermatological patients: the Freiburg Questionnaire on attitudes to naturopathy (FAN). Forschende Komplementarmedizin 1999;6(Suppl 2):26-9. [10352379] - PubMed
Bahmer 1991
-
- Bahmer FA, Schubert PH-J. Quantification of the extent and the severity of atopic dermatitis: the ADASI score. Archives of Dermatology 1991;127(8):1239-1240. - PubMed
Barnetson 2002
Batchelor 2010
-
- Batchelor JM, Grindlay DJ, Williams HC. What's new in atopic eczema? An analysis of systemic reviews published in 2008 and 2009. Clinical & Experimental Dermatology 2010;35(8):823-7. [PMID: 20649899] - PubMed
Bayles 2009
-
- Bayles B, Usatine R. Evening Primrose Oil. American Family Physician 2009;80(12):1405-1408. [MEDLINE: ] - PubMed
Belch 2000
-
- Belch JJ, Hill A. Evening primrose oil and borage oil in rheumatologic conditions. American Journal of Clinical Nutrition 2000;71(1 Suppl):352S-6S. [MEDLINE: ] - PubMed
Beswick 2011
-
- Beswick T, Morton J, Allen P. Report of two cases of chronic atopic dermatitis treated with adalimubab [P1318]. Journal of American Academy of Dermatology 2011;64(2 Suppl 1):AB60. [PIIS19096221001354X]
Bos 2010
-
- Bos JD, Brenninkmeijer EE, Schram ME, Middelkamp-Hup MA, Spuls PI, Smitt JH. Atopic eczema or atopiform dermatitis. Experimental Dermatology Apr 2010;19(4):325-31. [20100192] - PubMed
Charman 2000
-
- Charman CR, Morris AD, Williams HC. Topical corticosteroid phobia in patients with atopic eczema. British Journal of Dermatology 2000;142(5):931-6. [10809850] - PubMed
Cork 2009
-
- Cork MJ, Danby SG, Vasilopoulos Y, Hadgraft J, Lane ME, Moustafa M, et al. Epidermal Barrier Dysfunction in Atopic Dermatitis. Journal of Investigative Dermatology 2009;129(8):1892-908. [MEDLINE: ] - PubMed
Costa 1989
-
- Costa C, Rilliet A, Nicolet M and Surat JH. Scoring Atopic Dermatitis: The Simpler the Better? Acta Dermato-Venereologica 1989;69:41-45. [PMID: PMID: 2563607 ] - PubMed
Diezel 1993
-
- Diezel W, Schulz E, Shanks M, Heise H. Plant oils: topical application and anti-inflammatory effects (Croton oil test) [Pflanzliche ole: ausserliche anwendung und antientzundliche wirkungen (crotonol-test)]. Dermatologische Monatsschrift 1993;179(5):173-6.
Dove 1999
-
- Dove D, Johnson P. Oral evening primrose oil: its effect on length of pregnancy and selected intrapartum outcomes in low-risk nulliparous women. Journal of Nurse-Midwifery 1999;44(3):320-4. [MEDLINE: 10380450 ] - PubMed
Draelos 2008
-
- Draelos ZD. The effect of ceramide-containing skin care products on eczema resolution duration. Cutis 2008;81(1):87-91. - PubMed
Draelos 2011
-
- Draelos ZD, Koutatsu B, Butcher D, Billhimer W. Delivery of a pseudoceramide to atopic skin is related to objective and subjective improvement [P1306]. Journal of the American Academy of Dermatology 2011;64(2 Suppl 1):AB57.
Eisenberg 1998
-
- Eisenberg DM, David RB, Ettner SL, Appel S, Wilkey S, Van Rompay M, et al. Trends in alternative medicne use in the United States 1990-1997: results of a follow-up national survey. Journal of the American Medical Association 1998;280(18):1569-75. [EMBASE: 1998386275] - PubMed
Ellis 2002
-
- Ellis CN, Drake LA, Prendergast MM, Abramovits W, Boguniewitcz M, Daniel CR, et al. Cost of atopic dermatitis and eczema in the United States. Journal of the American Academy of Dermatology 2002;46(3):361-70. [11862170] - PubMed
Ernst 2000
-
- Ernst E. The usage of complementary therapies by dermatological patients: a systematic review. British Journal of Dermatology 2000;142(5):857-61. [MEDLINE: ] - PubMed
Finnigan 1991
-
- Finnigan MD. The Centre for the Study of Complementary Medicine: an attempt to understand its popularity through psychological, demographic and operational criteria. Complementary Medical Research 1991;5(2):83-8.
Friedmann 2002
-
- Friedmann PS. The pathogenesis of atopic eczema. Hospital Medicine 2002;63(11):653-6. - PubMed
Gfesser 1997
-
- Gfesser M, Abeck D, Rugemer J, Schreiner V, Stab F, Disch R, et al. The early phase of epidermal barrier regeneration is faster in patients with atopic eczema. Dermatology 1997;195(4):332-6. [MEDLINE: ] - PubMed
Hanifin 1980
-
- Hanifin J, Rajka G. Diagnostic features of atopic dermatitis. Acta Dermato-Venereologica 1980;92:44-7.
Herd 1996
-
- Herd RM, Tidman MJ, Prescot RJ, Hunter JA. The cost of atopic eczema. British Journal of Dermatology 1996;135(1):20-3. [PMID: ] - PubMed
Higgins 2011
-
- Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane-handbook.org.
Hoare 2000
Horrobin 2000
-
- Horrobin DF. Essential fatty acid metabolism and its modification in atopic eczema. American Journal of Clinical Nutrition 2000;71(1 Suppl):367S-72S. [MEDLINE: ] - PubMed
Imokawa 1991
-
- Imokawa G, Abe A, Jin K, Higaki Y, Kawashima M, Hidano A. Decreased level of ceramides in stratum corneum of atopic dermatitis: an etiologic factor in atopic dry skin. Journal of Investigative Dermatology 1991;96(4):523-6. [MEDLINE: ] - PubMed
Jellin 2005
-
- Jellin JM, editorial staff at Therapeutic Research Center. Natural Medicines: Comprehensive Database (Natural Medicines). 7th edition. Stockton CA: Pharmacist's Letter / Prescriber's Letter, 2005. [ISBN 0974706221]
Jensen 1990
-
- Jensen P. Use of alternative medicine by patients with atopic dermatitis and psoriasis. Acta Dermato-Venereologica 1990;70(5):421-4. [MEDLINE: ] - PubMed
Johansson 2001
-
- Johansson SG, Hourihane JO, Bousquet J, Bruijnzeel-Koomen C, Dreborg S, Haahteela T, et al. A revised nomenclature for allergy. An EAACI position statement from the EAACI nomenclature task force. Allergy 2001;56(9):813-24. [11551246] - PubMed
Johnson 1984
-
- Johnson ML, Johnson KG, Engel A. Prevalence, morbidity and cost of dermatological diseases. Journal of the American Academy of Dermatology 1984;11(5 Pt 2):930-6. [MEDLINE: ] - PubMed
Kramer 2012
Kubica 2011
-
- Kubica E, Ezzedine K, Lalanne N, Dartial Y, Taieb A, Milpied B. Oral alitretinoin in chronic refactory hand eczema: a "real life" case-series of 12 patients. European Journal of Dermatology 2011;21(3):454-6. [MEDLINE: ] - PubMed
Larsen 2002
-
- Larsen FS, Hanifin JM. Epidemiology of atopic dermatitis. Immunology & Allergy Clinics of North America 2002;22(1):1-24. [EMBASE: 2002061960]
Leung 2003
-
- Leung DY, Bieber T. Atopic dermatitis. Lancet 2003;361(9352):151-60. [MEDLINE: ] - PubMed
Leung 2008
-
- Leung DYM, Eichenfield LF and Bogunlewicz M. Atopic dermatitis (atopic eczema). In: Wolff K, Goldsmith LA, Katz SI, Gilchrest BA, Paller A and Leffel DJ, editors(s). Fitzpatrick's Dermatology in General Medicine. 7th edition. Vol. 1. NYC: McGraw=Hill, 2008:146-158. [ISBN = 9780071466905]
Lewis Jones 1995
-
- Lewis-Jones MS, Finlay AY. The Childrens' Dermatology Life Quality Index (CDLQI): initial validation and practical use.. British Journal of Dermatology 1995;132(6):942-9. [MEDLINE: ] - PubMed
Manku 1982
-
- Manku MS, Horrobin DF, Morse NL, Kyte V, Jenkins K, Wright S, et al. Reduced levels of prostaglandin precursors in the blood of atopic patients: defective delta-6-desaturated function as a biochemical basis for atopy. Prostaglandins Leukotrienes & Medicine 1982;9(6):615-28. [MEDLINE: ] - PubMed
Matsumoto 2000
-
- Matsumoto M, Sugiura H, Uehara M. Skin barrier function in patients with completely healed atopic dermatitis. Journal of Dermatological Science 2000;23(3):178-82. [MEDLINE: ] - PubMed
McNally 1998
-
- McNally NJ, Williams HC, Smallman-Raynor M, Lewis S, Venn A, Britton J. Atopic eczema and domestic water hardness. Lancet 1998;352(9127):527-31. [MEDLINE: ] - PubMed
McNally 2001
-
- McNally NJ, Williams HC, Phillips DR. Atopic eczema and the home environment. British Journal of Dermatology 2001;145(5):730-6. [MEDLINE: ] - PubMed
Melnik 1992
-
- Melnik B, Plewig G. Are disturbances of omega-6-fatty acid metabolism involved in the pathogenesis of atopic dermatitis? Acta Dermato-Venereologica 1992;176:77-85. [MEDLINE: 1476044 ] - PubMed
Moore 2009
-
- Moore EJ, Williams A, Manias E, Varigos G, Donath S. Eczema workshops reduce severity of childhood atopic eczema. Australasian Journal of Dermatology 2009;50(2):100-6. [MEDLINE: ] - PubMed
Morse 1989
-
- Morse PF, Horrobin DF, Manku MS, Stewart JC, Allen R, Littlewood S, et al. Meta-analysis of placebo-controlled studies of the efficacy of Epogam in the treatment of atopic eczema. Relationship between plasma essential fatty acid changes and clinical response. British Journal of Dermatology 1989;121(1):75-90. [MEDLINE: ] - PubMed
Morse 2006
-
- Morse NL, Clough PM. A meta-analysis of randomized, placebo-controlled clinical trials of Efamol evening primrose oil in atopic eczema. Where do we go from here in light of more recent discoveries? Current Pharmaceutical Biotechechnology 2006;7(6):503-24. [MEDLINE: ] - PubMed
Nankervis 2010
NHIS 2007
-
- National Health Interview Survey (NHIS). 2007 CAM Use Data. http://nccam.nih.gov/news/camstats/ (accessed 28 August 2012).
Nordstrom 1995
-
- Nordstrom DC, Honkanen VE, Nasu Y, Antila E, Friman C, Konttinen YT. Alpha-linolenic acid in the treatment of rheumatoid arthritis. A double-blind, placebo-controlled and randomized study: flaxseed vs. safflower seed. Rheumatology International 1995;14(6):231-4. [MEDLINE: ] - PubMed
Pastar 2005
-
- Pastar Z, Lipozencic J, Ljubojevic S. Etiopathogenesis of atopic dermatitis-an overview. Acta Dermatovenerologica Croatica 2005;13(1):54-62. [MEDLINE: ] - PubMed
Patel 2011
Phinney 1994
-
- Phinney S. Potential risk of prolonged gamma-linolenic acid use. Annals of Internal Medicine 1994;120(8):692. [MEDLINE: ] - PubMed
Plötz 2010
-
- Plötz SG, Ring J. What's new in atopic eczema? Expert Opinion on Emerging Drugs 2010;15(2):249-67. [MEDLINE: ] - PubMed
Poyner 2001
-
- Poyner T. PCDS atopic eczema guidelines optimise GP management. Guidelines in Practice 2001;4(1):33-43.
Puri 2007
-
- Puri BK. The safety of evening primrose oil in epilepsy. Prostaglandins Leukotrienes & Essential Fatty Acids 2007;77(2):101-3. [PMID: ] - PubMed
Rabahi 2010
-
- Rabahi MF, Ferreira AA, Madeira JG, Galvao PM, Pinto SA. Lipoid pneumonia secondary to long-term use of evening primrose oil. Jornal Brasileiro De Pneumologia 2010;36(5):657-61. [PMID: ] - PubMed
Rajka 1989
-
- Rajka G. Essential aspects of atopic dermatitis. Berlin: Springer Verlag, 1989.
Reid 1995
-
- Reid P, Lewis-Jones MS. Sleep difficulties and their management in preschoolers with atopic eczema. Clinical & Experimental Dermatology 1995;20(1):38-41. [7671394] - PubMed
Riaz 2009
-
- Riaz A, Khan RA, Ahmed SP. Assessment of anticoagulant effect of evening primrose oil. Pakistan Journal of Pharmaceutical Sciences 2009;22(4):355-9. [MEDLINE: ] - PubMed
Roberts 2010
-
- Roberts H, Orchard D. Methotrexate is a safe and effective treatment for paediatric discoid (nummular) eczema: a case series of 25 children. Australasian Journal of Dermatology 2010;51(2):128-30. [PMID: ] - PubMed
Saito 2005
-
- Saito, H. Much Atopy about the Skin: Genome-Wide Molecular Analysis of Atopic Eczema. International Archives of Allergy & Immunology 2005;137(4):319-25. [15970641] - PubMed
Sampson 1992
-
- Sampson HA. Atopic dermatitis. Annals of Allergy 1992;69(6):469-79. [1471777] - PubMed
Schmitt 2007
Schram 2010
-
- Schram ME, Tedja AM, Spijker R, Bos JD, Williams HC, Spurls PI. Is there a rural/urban gradient in prevalence of eczema? A systematic review. British Journal of Dermatology 2010;162(5):964-73. [PMID: ] - PubMed
Schultz‐Larsen 1993
-
- Schultz Larsen F. Atopic dermatitis: a genetic-epidemiological study in a population-based twin sample. Journal of the American Academy of Dermatology 1993;28(5 Pt 1):719-23. [8496415] - PubMed
SCORAD index 1993
-
- Severity scoring of atopic dermatitis: the SCORAD index. Consensus Report of the European Task Force on Atopic Dermatitis. Dermatology 1993;186(1):23-31. - PubMed
Shahidi 1997
-
- Shahidi F, Amarowicz R, He Y, Wettasinghe M. Antioxidant activity of phenolic extracts of evening primrose (Oenothera biennis): a preliminary study. Journal of Food Lipids 1997;4:75-86.
Sheehan 1992
-
- Sheehan MP, Rustin MH, Atherton DJ, Buckley C, Harris DW, Brostoff J, et al. Efficacy of traditional Chinese herbal therapy in adult atopic dermatitis. Lancet 1992;340(8810):13-7. [MEDLINE: ] - PubMed
Su 1997
Terreehorst 2002
-
- Terreehorst I, Duivenvoorden HJ, Temples-Pavlica Z, Oosting AJ, Monchy JG, Bruijnzeel-Koomen CA, et al. The unfavourable effects of concomitant asthma and sleeplessness due to the atopic eczema/dermatitis syndrome (AEDS) on quality of life in subjects allergic to house-dust mites. Allergy 2002;57(10):919-25. [MEDLINE: ] - PubMed
Thestrup‐Pedersen 2003
-
- Thestrup-Pedersen, K. Tacrolimus treatment of atopic eczema/dermatitis syndrome. Current Opinion in Allergy & Clinical Immunology 2003;3(5):359-62. [14501435] - PubMed
Tofte 2001
-
- Tofte SJ, Hanifin JM. Current management and therapy of atopic dermatitis. Journal of the American Academy of Dermatology 2001;44(1 Suppl):S13-16. [MEDLINE: ] - PubMed
Triebskorn 1989
-
- Triebskorn A, Drosner M. Methods of alternative medicine treatment assessed by patients with allergies and patients with chronic skin diseases ["Alternativ-medizinische" Behandlungsmethoden in der Beurteilung vonAllergikern und chronisch Hautkranken]. H+G Zeitschrift für Hautkrankheiten 1989;64(6):487-94. [MEDLINE: ] - PubMed
van Gool 2003
-
- Gool CJ, Thijs C, Henquet CJ, Houwelingen AC, Dagnelie PC, Schrander J, et al. Gamma-linolenic acid supplementation for prophylaxis of atopic dermatitis - a randomized controlled trial in infants at high familial risk. American Journal of Clinical Nutrition 2003;77(4):943-51. [PMID: ] - PubMed
van Odijk 2003
-
- Odijk J, Kull I, Borres MP, Brandtzaeg P, Edberg U, Hanson LA, et al. Breastfeeding and allergic disease: a multidisciplinary review of the literature (1966-2001) on the mode of early feeding in infancy and its impact on later atopic manifestations. Allergy 2003;58(9):833-43. [MEDLINE: ] - PubMed
Wang 2007
-
- Wang IJ, Guo YL, Weng HJ, Hsieh WS, Chuang YL, Lin SJ, Chen PC. Environmental risk factors for early infantile atopic dermatitis. Pediatric Allergy & Immunology 2007;18(5):441-7. [PMID: ] - PubMed
Weller 2009
-
- Weller RPJB, Hunter JAA, Savin JA, Dahl MV. Eczema and Dermatitis. chapter in Clinical Dermatology 2009;Chapter 7:79-103. [DOI: ] [SN: 9781444300086.ch7]
Williams 1994
Williams 2000
-
- Williams HC, Wüthrich B. The natural history of atopic dermatitis. In: Williams HC, editors(s). Atopic Dermatitis. Cambridge University Press, 2000:49-59. [978-0521570756]
Williams 2008
-
- Williams HC, Grindlay DJ. What's new in atopic eczema? An analysis of the clinical significance of systemic reviews on atopic eczema published in 2006 and 2007. Clinical & Experimental Dermatology 2008;33(6):685-8. [PMID: ] - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

