Antiplatelet drugs for polycythaemia vera and essential thrombocythaemia
- PMID: 23633335
- PMCID: PMC11365094
- DOI: 10.1002/14651858.CD006503.pub3
Antiplatelet drugs for polycythaemia vera and essential thrombocythaemia
Abstract
Background: Polycythaemia vera and essential thrombocythaemia are chronic Philadelphia-negative myeloproliferative neoplasms that increase the risk of arterial and venous thrombosis, as well as bleeding. In addition to the different therapeutic strategies available, an antiplatelet drug is often used to reduce thrombotic risk.
Objectives: To quantify the benefit and harm of antiplatelet drugs for long-term primary and secondary prophylaxis of arterial and venous thrombotic events in patients with polycythaemia vera or essential thrombocythaemia.
Search methods: We searched the Cochrane Central Register of Controlled Trials (CENTRAL), The Cochrane Library (Issue 1 2012), MEDLINE (1966 to 2012), and EMBASE (1980 to 2012), as well as online registers of ongoing trials and conference proceedings. The date of the last search was October 2012.
Selection criteria: We included all randomised controlled trials (RCTs) comparing long-term (>6 months) use of an antiplatelet drug versus placebo or no treatment in participants with polycythaemia vera or essential thrombocythaemia, as diagnosed by established international criteria, with data for at least one of the selected outcomes.
Data collection and analysis: Using a pre-defined extraction form, two review authors independently screened results, extracted data, and assessed quality. We planned to analyse the following outcomes: mortality from arterial and venous thrombotic events (primary efficacy outcome), mortality from bleeding episodes (primary safety outcome), fatal and non-fatal arterial thrombotic events, fatal and non-fatal venous thrombotic events, micro-circulation events, transient neurological and ocular manifestations, major and minor bleeding episodes, and all-cause mortality and any adverse events. We based quantitative analysis of outcome data on an intention-to-treat principle. We used the pooled odds ratio (OR) with 95% confidence interval (CI) with a fixed-effect model (Mantel-Haenszel) to estimate the overall treatment effect.
Main results: We identified no new studies from the updated searches. We included in this review two RCTs for a total of 630 participants. Both RCTs included participants with an established diagnosis of polycythaemia vera and with no clear indication or contraindication to aspirin therapy. We judged both studies to be of moderate quality. Published data from both studies were insufficient for a time-to-event data analysis and for some of the primary and secondary outcomes that we planned. The use of low-dose aspirin, compared with placebo, was associated with a lower risk of fatal thrombotic events (although this benefit was not statistically significant (OR 0.20, 95% CI 0.03 to 1.14; P = 0.07). No data on mortality from bleeding episodes were available. A non-significant benefit of aspirin was shown for all-cause mortality (OR 0.46, 95% CI 0.21 to 1.01; P = 0.05). No increase in the risk of major bleeding was reported in participants taking aspirin compared with those given placebo (OR 0.99, 95% CI 0.23 to 4.36; P = 0.99), and a non-significant increase with aspirin treatment was shown for minor bleeding (OR 1.85, 95% CI 0.90 to 3.79; P = 0.09). No published studies have reported findings in participants with essential thrombocythaemia or in the study of other antiplatelet drugs.
Authors' conclusions: For patients with polycythaemia vera who have no clear indication or contraindication to aspirin therapy, available evidence suggests that the use of low-dose aspirin, when compared with no treatment, is associated with a statistically non-significant reduction in the risk of fatal thrombotic events and all-cause mortality, without an increased risk of major bleeding.
Conflict of interest statement
None known.
Figures

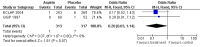
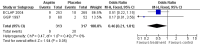
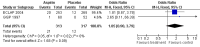
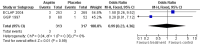




Update of
-
Antiplatelet drugs for polycythaemia vera and essential thrombocythaemia.Cochrane Database Syst Rev. 2008 Apr 16;(2):CD006503. doi: 10.1002/14651858.CD006503.pub2. Cochrane Database Syst Rev. 2008. Update in: Cochrane Database Syst Rev. 2013 Apr 30;(4):CD006503. doi: 10.1002/14651858.CD006503.pub3. PMID: 18425953 Updated.
References
References to studies included in this review
ECLAP 2004 {published data only}
-
- Landolfi R, Marchioli R, Kutti J, Gisslinger H, Tognoni G, Patrono C, et al. Efficacy and safety of low‐dose aspirin in polycythaemia vera. New England Journal of Medicine 2004;350:114‐24. - PubMed
GISP 1997 {published data only}
-
- Gruppo Italiano Studio Policitemia Vera (GISP). Low‐dose aspirin in polycythaemia vera: a pilot study. British Journal of Haematology 1997;97:453‐6. - PubMed
References to studies excluded from this review
Finelli 1991 {published data only}
-
- Finelli C, Palareti G, Poggi M, Torricelli P, Vianelli N, Fiacchini M, et al. Ticlopidine lowers plasma fibrinogen in patients with polycythaemia rubra vera and additional thrombotic risk factors. Acta Haematologica 1991;85:113‐8. - PubMed
Tartaglia 1986 {published data only}
-
- Tartaglia AP, Goldberg JD, Berck PD, Wasserman LR. Adverse effects of antiaggregant platelet therapy in the treatment of polycythaemia vera. Seminars in Hematology 1986;23:172‐6. - PubMed
References to ongoing studies
ISCLAP {published data only}
-
- Clopidogrel and Aspirin for the Treatment of Polycythemia Vera (ISCLAP). NCT00940784. http://clinicaltrials.gov last search: 26 February 2012.
Additional references
ACCP 2012
Alvarez‐Larran 2010
-
- Alvarez‐Larran A, Cervantes F, Pereira A, Arellano‐Rodrigo E, Perez‐Andreu V, Hernandez‐Boluda JC, et al. Observation versus antiplatelet therapy as primary prophylaxis for thrombosis in low‐risk essential thrombocythemia. Blood 2010;116:1205‐10. - PubMed
Ania 1994
-
- Ania BJ, Suman VJ, Sobell JL, et al. Trends in the incidence of polycythemia vera among Olmsted County, Minnesota residents, 1935‐1989. American Journal of Hematology 1994;47:89‐93. - PubMed
ATC 2002
Campbell 2005
-
- Campbell PJ, Green AR. Management of polycythaemia vera and essential thrombocythaemia. Hematology/Education Program of the American Society of Hematology 2005;1:201‐8. - PubMed
Cortelazzo 1990
-
- Cortelazzo S, Viero P, Finazzi G, D'Emilio A, Rodeghiero F, Barbui T. Incidence and risk factors for thrombotic complications in a historical cohort of 100 patients with essential thrombocythaemia. Journal of Clinical Oncology 1990;8:556‐62. - PubMed
Cortelazzo 1995
-
- Cortelazzo S, Finazzi G, Ruggeri M, Vestri O, Galli M, Rodeghiero F, et al. Hydroxyurea for patients with essential thrombocythaemia and high risk of thrombosis. New England Journal of Medicine 1995;332:1132‐6. - PubMed
Deeks 2011
-
- Deeks JJ, Higgins JPT, Altman DG (editors). Chapter 9: Analysing data and undertaking meta‐analyses. Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. www.cochrane‐handbook.org.
Egger 1997
Finazzi 2005
-
- Finazzi G, Caruso V, Marchioli R, Capnist G, Chisesi T, Finelli C, et al. Acute leukaemia in polycythaemia vera: an analysis of 1638 patients enrolled in a prospective observational study. Blood 2005;105:2664‐70. - PubMed
GISP 1995
-
- Gruppo Italiano Studio Policitemia. Polycythemia vera: the natural history of 1213 patients followed for 20 years. Annals of Internal Medicine 1995;123:656‐64. - PubMed
Harrison 2005
-
- Harrison CN, Campbell PJ, Buck G, Wheatley K, East CL, Bareford D, et al. Hydroxyurea compared with anagrelide in high‐risk essential thrombocythemia. New England Journal of Medicine 2005;353(1):33‐45. - PubMed
Higgins 2003
Higgins 2011a
-
- Higgins JPT, Deeks JJ (editors). Chapter 7: Selecting studies and collecting data. Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. www.cochrane‐handbook.org.
Higgins 2011b
-
- Higgins JPT, Altman DG (editors). Chapter 8: Assessing risk of bias in included studies. Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011 www.cochrane‐handbook.org.
Landolfi 1992
-
- Landolfi R, Ciabattoni G, Pugliese F, et al. Increased thromboxane biosynthesis in patients with polycythaemia vera: evidence for aspirin‐suppressible platelet activation in vivo. Blood 1992;80:1965‐71. - PubMed
Landolfi 1997
-
- Landolfi R, Marchioli R. European Collaboration on Low‐dose Aspirin in Polycythemia Vera (ECLAP): a randomised trial. Seminars in Thrombosis and Haemostasis 1997;23:473‐8. - PubMed
Lau 2006
Maclure 1987
-
- Maclure M, Willett WC. Misinterpretation and misuse of the kappa statistic. American Journal of Epidemiology 1987;126:161‐9. - PubMed
Marchioli 2005
-
- Marchioli R, Finazzi G, Landolfi R, Kutti J, Gisslinger H, Patrono C, et al. Vascular and neoplastic risk in a large cohort of patients with polycythaemia vera. Journal of Clinical Oncology 2005;23:2224‐32. - PubMed
Mesa 1999
-
- Mesa RA, Silverstein MN, Jacobsen SJ, et al. Population‐based incidence and survival figures in essential thrombocythemia and agnogenic myeloid metaplasia: an Olmsted County Study, 1976–1995. American Journal of Hematology 1999;61:10‐15. - PubMed
Pardanani 2011
-
- Pardanani A, Tefferi A. Targeting myeloproliferative neoplasms with JAK inhibitors. Curr Opin Hematol 2011;18:105‐110. - PubMed
Parmar 1998
-
- Parmar MK, Torri V, Stewart L. Extracting summary statistics to perform meta‐analyses of the published literature for survival endpoints. Statistics in Medicine 1998;17:2815‐34. - PubMed
Passamonti 2004
-
- Passamonti F, Rumi E, Pungolino E, et al. Life expectancy and prognostic factors for survival in patients with polycythaemia vera and essential thrombocythaemia. American Journal of Medicine 2004;117:755‐61. - PubMed
PRISMA
-
- Moher D, Liberati A, Tetzlaff J, Altman DG, PRISMA Group. Preferred reporting items for systematic reviews and meta‐analyses: the PRISMA statement.. J Clin Epidemiol 2009;62:1006‐12. - PubMed
RevMan 5 [Computer program]
-
- The Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager (RevMan). Version 5.1. Copenhagan: The Nordic Cochrane Centre, The Cochrane Collaboration, 2011.
Rocca 1995
-
- Rocca B, Ciabattoni G, Tartaglione R, et al. Increased thromboxane biosynthesis in essential thrombocythaemia. Thrombosis and Haemostasis 1995;74:1225‐30. - PubMed
Sterne 2011
-
- Sterne JAC, Egger M, Moher D (editors). Chapter 10: Addressing reporting biases. Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011].. The Cochrane Collaboration, 2011 www.cochrane‐handbook.org.
WHO 2002
-
- Vardiman JW, Harris NL, Brunning RD. The World Health Organization (WHO) classification of the myeloid neoplasms. Blood 2002;100:2292‐302. - PubMed
References to other published versions of this review
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

