Combination formoterol and budesonide as maintenance and reliever therapy versus current best practice (including inhaled steroid maintenance), for chronic asthma in adults and children
- PMID: 23633340
- PMCID: PMC10357488
- DOI: 10.1002/14651858.CD007313.pub3
Combination formoterol and budesonide as maintenance and reliever therapy versus current best practice (including inhaled steroid maintenance), for chronic asthma in adults and children
Abstract
Background: Traditionally inhaled treatment for asthma has used separate preventer and reliever therapies. The combination of formoterol and budesonide in one inhaler has made possible a single inhaler for both prevention and relief of symptoms (single inhaler therapy or SiT).
Objectives: To assess the efficacy and safety of budesonide and formoterol in a single inhaler for maintenance and reliever therapy in asthma compared with maintenance with inhaled corticosteroids (ICS) (alone or as part of current best practice) and any reliever therapy.
Search methods: We searched the Cochrane Airways Group trials register in February 2013.
Selection criteria: Parallel, randomised controlled trials of 12 weeks or longer in adults and children with chronic asthma. Studies had to assess the combination of formoterol and budesonide as SiT, against a control group that received inhaled steroids and a separate reliever inhaler.
Data collection and analysis: We used standard methodological procedures expected by The Cochrane Collaboration.
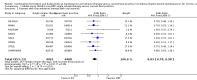
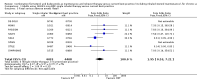
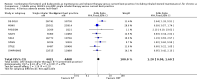
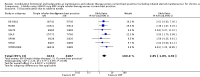
Main results: We included 13 trials involving 13,152 adults and one of the trials also involved 224 children (which have been separately reported). All studies were sponsored by the manufacturer of the SiT inhaler. We considered the nine studies assessing SiT against best practice to be at a low risk of selection bias, but a high risk of detection bias as they were unblinded.In adults whose asthma was not well-controlled on ICS, the reduction in hospital admission with SiT did not reach statistical significance (Peto odds ratio (OR) 0.81; 95% confidence interval (CI) 0.45 to 1.44, eight trials, N = 8841, low quality evidence due to risk of detection bias in open studies and imprecision). The rates of hospital admission were low; for every 1000 people treated with current best practice six would experience a hospital admission over six months compared with between three and eight treated with SiT. The odds of experiencing exacerbations needing treatment with oral steroids were lower with SiT compared with control (OR 0.83; 95% CI 0.70 to 0.98, eight trials, N = 8841, moderate quality evidence due to risk of detection bias). For every 100 adults treated with current best practice over six months, seven required a course of oral steroids, whilst for SiT there would be six (95% CI 5 to 7). The small reduction in time to first severe exacerbation needing medical intervention was not statistically significant (hazard ratio (HR) 0.94; 95% CI 0.85 to 1.04, five trials, N = 7355). Most trials demonstrated a reduction in the mean total daily dose of ICS with SiT (mean reduction was based on self-reported data from patient diaries and ranged from 107 to 385 µg/day). Withdrawals due to adverse events were more common in people treated with SiT (OR 2.85; 95% CI 1.89 to 4.30, moderate quality evidence due to risk of detection bias).Three studies including 4209 adults compared SiT with higher dose budesonide maintenance and terbutaline for symptom relief. The studies were considered as low risk of bias. The run-in for these studies involved withdrawal of LABA, and patients were recruited who were symptomatic during run-in. The reduction in the odds of hospitalisation with SiT compared with higher dose ICS did not reach statistical significance (Peto OR; 0.56; 95% CI 0.28 to 1.09, moderate quality evidence due to imprecision). Fewer patients on SiT needed a course of oral corticosteroids (OR 0.54; 95% CI 0.45 to 0.64, high quality evidence). For every 100 adults treated with ICS over 11 months, 18 required a course of oral steroids, whilst for SiT there would be 11 (95% CI 9 to 12). Withdrawals due to adverse events were more common in people treated with SiT (OR 0.57; 95% CI 0.35 to 0.93, high quality evidence).One study included children (N = 224), in which SiT was compared with higher dose budesonide. There was a significant reduction in participants who needed an increase in their inhaled steroids with SiT, but there were only two hospitalisations for asthma and no separate data on courses of oral corticosteroids. Less inhaled and oral corticosteroids were used in the SiT group and the annual height gain was also 1 cm greater in the SiT group, (95% CI 0.3 cm to 1.7 cm).The results for fatal serious adverse events were too rare to rule out either treatment being harmful. There was no significant difference found in non-fatal serious adverse events for any of the comparisons.
Authors' conclusions: Single inhaler therapy has now been demonstrated to reduce exacerbations requiring oral corticosteroids against current best practice strategies and against a fixed higher dose of inhaled steroids. The strength of evidence that SiT reduces hospitalisation against these same treatments is weak. There were more discontinuations due to adverse events on SiT compared to current best practice, but no significant differences in serious adverse events. Our confidence in these conclusions is limited by the open-label design of the trials, and by the unknown adherence to treatment in the current best practice arms of the trials.Single inhaler therapy can reduce the risk of asthma exacerbations needing oral corticosteroids in comparison with fixed dose maintenance ICS and separate relief medication. The reduced odds of exacerbations with SiT compared with higher dose ICS should be viewed in the context of the possible impact of LABA withdrawal during study run-in. This may have made the study populations more likely to respond to SiT.Single inhaler therapy is not currently licensed for children under 18 years of age in the United Kingdom and there is currently very little research evidence for this approach in children or adolescents.
Conflict of interest statement
None known.
Figures







































Update of
-
Combination formoterol and budesonide as maintenance and reliever therapy versus inhaled steroid maintenance for chronic asthma in adults and children.Cochrane Database Syst Rev. 2009 Apr 15;(2):CD007313. doi: 10.1002/14651858.CD007313.pub2. Cochrane Database Syst Rev. 2009. Update in: Cochrane Database Syst Rev. 2013 Apr 30;(4):CD007313. doi: 10.1002/14651858.CD007313.pub3. PMID: 19370682 Free PMC article. Updated.
References
References to studies included in this review
DE‐SOLO {published and unpublished data}
-
- AstraZeneca (D5890L00011). A comparison of Symbicort® single inhaler therapy (Symbicort® Turbuhaler® 160/4.5 mcg, 1 inhalation b.i.d. plus as‐needed) and conventional best practice for the treatment of persistent asthma in adults ‐ a 26‐week, randomised, open‐label, parallel‐group, multicentre study. http://www.astrazenecaclinicaltrials.com/_mshost800325/content/clinical‐... Accessed December 12th 2012. [NCT00252863]
MONO {published data only}
-
- AstraZeneca (D5890L00008). MONO: Symbicort single inhaler therapy and conventional best standard treatment for the treatment of persistent asthma in adolescents and adults. clinicaltrials.gov 2006:http://www.clinicaltrials.gov/ct/show/NCT00242411 [Accessed 12/04/2006].
PASSION {published data only}
-
- AstraZeneca. A comparison of Symbicort single inhaler therapy (Symbicort Turbuhaler160/4.5 mg, 1 inhalation b.i.d. plus as needed) and conventional best practice for the treatment of persistent asthma in adults ‐ a 26‐week, randomised, open‐label,parallel‐group, multicentre study – PASSION Study [D5890L00016]. http://www.astrazenecaclinicaltrials.com/_mshost800325/content/clinical‐... Accessed July 2012. [NCT00628758]
Riemersma (NCT00235911) {published and unpublished data}
-
- AstraZeneca (BN‐00S‐0011). Symbicort single inhaler therapy for asthma in a general practice setting. http://www.astrazenecaclinicaltrials.com/_mshost800325/content/clinical‐... Accessed Jluy 2012. [NCT00235911]
SALTO {published data only}
-
- AstraZeneca (D5890L00009). SALTO ‐ symbicort single inhaler therapy use in adolescent adults and adults with persistent asthma. www.clinicaltrials.gov 2006:http://www.clinicaltrials.gov/ct/show/NCT00290264 [Accessed 22/02/2008].
Scicchitano 2004 {published data only}
-
- Scicchitano R, Aalbers R, Ukena D, Manjra A, Fouquert L, Centann S, et al. Efficacy and safety of budesonide/formoterol single inhaler therapy versus a higher dose of budesonide in moderate to severe asthma. Current Medical Research and Opinion 2004;20(9):1403‐18. - PubMed
SOLO {published data only}
-
- Sears MR, Boulet L‐P, Laviolette M, FitzGerald JM, Bai R, SmiljanicGeorijev N, et al. Budesonide/formoterol maintenance and reliever therapy for asthma compared to conventional best practice a randomised real life study. European Respiratory Journal 2006;28(Suppl 50):613s.
-
- Sears MR, Boulet L‐P, Laviolette M, FitzGerald JM, Bai TR, Kaplan A, et al. Budesonide/formoterol maintenance and reliever therapy: impact on airway inflammation in asthma. European Respiratory Journal 2008:Epub: doi: 10.1183/09031936.00104007. - PubMed
Sovani 2008 {published data only}
SPAIN {published and unpublished data}
-
- AstraZeneca. A comparison of Symbicort single inhaler therapy (Symbicort Turbuhaler160/4.5 mcg, 1 inhalation b.i.d. plus as needed) and conventional best practice for the treatment of persistent asthma in adults ‐ a 26‐week, randomised,open‐label, parallel‐group, multicentre study. Study SPAIN [D5890L00010]. http://www.astrazenecaclinicaltrials.com/_mshost800325/content/clinical‐... Accessed July 2012.
-
- AstraZeneca. Symbicort single inhaler therapy vs conventional best practice for the treatment of persistent asthma in adults. http://clinicaltrials.gov/ct2/show/results/NCT00385593?sect=X30125#evnt Accessed December 12th 2012. [NCT00385593]
-
- Quirce S, Barcina C, Plaza V, Calvo E, Munoz M, Ampudia R, et al. A comparison of budesonide/formoterol maintenance and reliever therapy versus conventional best practice in asthma management in Spain. Journal of Asthma 2011;48:839‐47. - PubMed
STAY ‐ Adults {published and unpublished data}
-
- Bateman ED, Palmqvist M, Juniper EF, Zhu Y, Ekstrom T. Single inhaler therapy with budesonide/formoterol has superior efficacy to fixed‐dose budesonide/formoterol or a higher dose of budesonide alone. American Thoracic Society 100th International Conference, May 21‐26, 2004, Orlando. 2004.
-
- Bruce SA, Scherer YK. Maintenance and symptom relief with budesonide plus formoterol reduced severe asthma exacerbations. Evidence‐Based Nursing 2005;8(3):78. - PubMed
-
- Jönsson BG, Berggren FE, Svensson K, O'Byrne PM. Budesonide and formoterol in mild persistent asthma compared with doubling the dose of budesonide ‐ a cost‐effectiveness analysis. European Respiratory Journal. 2001; Vol. 18, issue Suppl 33:517s.
-
- Jönsson BG, Berggren FE, Svensson K, O'Byrne PM. Economic results of adding formoterol to budesonide in mild persistent asthma. European Respiratory Journal 2001; Vol. 18, issue Suppl 33:331s.
-
- O'Byrne PM. Acute asthma intervention: Insights from the STAY study. Journal of Allergy and Clinical Immunology 2007;119(6):1332‐6. - PubMed
STAY ‐ Children {published and unpublished data}
-
- Bisgaard H, Hultquist C. Budesonide/formoterol for maintenance and as needed ‐ a new approach to asthma management in children [Abstract]. European Respiratory Journal 2005;26(Suppl 49):Abstract No. 1060.
-
- Bisgaard H, Roux P, Bjamer D, Dymek A, Vermeulen JH, Hultquist C. Budesonide/formoterol maintenance plus reliever therapy ‐ a new strategy in pediatric asthma. Chest 2006;130(6):1733‐43. - PubMed
-
- O'Byrne PM. Acute asthma intervention: Insights from the STAY study. Journal of Allergy and Clinical Immunology 2007;119(6):1332‐6. - PubMed
-
- O'Byrne PM, Bisgaard H, Godard PP, Pistolesi M, Palmqvist M, Zhu Y, et al. Budesonide/formoterol combination therapy as both maintenance and reliever medication in asthma. American Journal of Respiratory and Critical Care Medicine 2005;171(2):129‐36. - PubMed
-
- SD‐039‐673. Efficacy and safety of budesonide/formoterol (Symbicort) Turbuhaler® as single therapy in patients with mild‐moderate asthma. Comparison with Symbicort Turbuhaler and Pulmicort® Turbuhaler as maintenance therapy, both complemented with Bricanyl® Turbuhaler (STAY). http://www.astrazenecaclinicaltrials.com 2006.
STEAM {published data only}
-
- Astrazenca (SD‐039‐0667). Efficacy and safety of Symbicort® Turbuhaler® as single therapy in patients with mild to moderate asthma ‐ STEAM (SD‐039‐0667). Astrazeneca Clinical Trials Register 2005:http://www.astrazenecaclinicaltrials.com (accessed 20th February 2008).
-
- Rabe KF, Pizzichini E, Stallberg B, Romero S, Balanzat AM, Atienza T, et al. Budisonide/formoterol in a single inhaler for maintenance and relief in mild‐to‐moderate asthma: A randomized, double‐blind trial. Chest 2006;129:245‐56. - PubMed
STYLE {published data only}
-
- AstraZeneca. STYLE ‐ A Comparison of Symbicort SMART (Symbicort Turbuhaler160/4,5 mcg, 1 inhalation b.i.d. plus as needed) and conventional best practice for the treatment of persistent asthma in adolescents and adults – a 26‐week, open‐labelled, parallel‐group, multicentre study. http://www.astrazenecaclinicaltrials.com/_mshost800325/content/clinical‐... Accessed July 2012. [NCT00252824]
-
- AstraZeneca (D5890L00014). STYLE ‐ symbicort single inhaler therapy vs. conventional therapy in treatment of persistent asthma. www.clinicaltrials.gov 2005:http://www.clinicaltrials.gov/ct/show/NCT00252824 [Accessed 22/02/2008].
SYMPHONIE {published data only}
-
- AstraZeneca. A comparison of Symbicort single inhaler therapy (Symbicort Turbuhaler 200/6 μg, 1 inhalation b.i.d. plus as needed) and conventional best practice for the treatment of persistent asthma in adolescents and adults – a 26‐week, randomised, open, parallel group multicentre study. [D5890L00005]. http://www.astrazenecaclinicaltrials.com/_mshost800325/content/clinical‐... Accessed July 2012. [NCT00259792]
References to studies excluded from this review
Balanzat 2004 {published data only}
-
- Balanzat A, Centanni S, Palmqvist M, Rabe K. Budesonide/formoterol single inhaler therapy reduces over reliance on rapid acting reliever medication [Abstract]. European Respiratory Journal 2004;24(Suppl 48):344s.
Bousquet 2007 {unpublished data only}
-
- Astrazeneca (D5890C00002). Efficacy and safety of Symbicort®Turbuhaler®160/4.5 mcg/inhalation, two inhalations twice daily plus as‐needed compared with Seretide™ Diskus™ 50/500 mcg/inhalation, one inhalation twice daily plus terbutaline Turbuhaler 0.4 mg/inhalation as‐needed ‐ a 6‐month, randomised, double‐blind, parallel‐group, active controlled, multinational phase IIIB study in adult and adolescent patients with persistent asthma. AstraZeneca Clinical Trials Register 2006, issue www.astrazenecaclinicaltrials.com (accessed 21/02/2008).
-
- Bousquet J, Boulet L‐P, Peters MJ, Magnussen H, Quiralte J, Martinez‐Aguilar NE, et al. Budesonide/formoterol for maintenance and relief in uncontrolled asthma vs. high‐dose salmeterol/fluticasone. Respiratory Medicine 2007;101(12):2437‐46. - PubMed
COMPASS {published and unpublished data}
-
- AstraZeneca. SYM/050/DEC2007. Data on File.
-
- Bleecker ER, Postma DS, Lawrance R, Meyers DA, Ambrose H, Goldman M. Effect of polymorphisms in the beta2‐adrenergic receptor gene (ADRB2) on response to long‐acting beta2‐agonist (LABA) therapy. Journal of Allergy and Clinical Immunology 2007;119(2):523.
-
- Buhl R, Vogelmeier C. Budesonide/formoterol maintenance and reliever therapy: a new treatment approach for adult patients with asthma. Current Medical Research and Opinion 2007;23(8):1867‐78. - PubMed
-
- Kuna P, Peters MJ, Buhl R. Budesonide/formoterol as maintenance and reliever therapy reduces asthma exacerbations a higher maintenance dose of budesonide/versus formoterol or salmeterol/fluticasone. European Respiratory Journal 2006;28(Suppl 50):205s.
COSMOS {published data only}
-
- Buhl R, Vogelmeier C. Budesonide/formoterol maintenance and reliever therapy: a new treatment approach for adult patients with asthma. Current Medical Research and Opinion 2007;23(8):1867‐78. - PubMed
-
- D'Urzo A, Vogeimeier C, Jaspal M, Merino JM, Boulet S. Symbicort (budesonide/formoterol) for both maintenance and relief reduces the exacerbation burden compared with titration of seretide (salmeterol/fluticasone) in patients with asthma, a real life study. American Thoracic Society International Conference; May 20‐25; San Diego, California. 2005:Poster G24.
-
- Johansson G, Andreasson EB, Larsson PE, Vogelmeier CF. Cost effectiveness of budesonide/formoterol for maintenance and reliever therapy versus salmeterol/fluticasone plus salbutamol in the treatment of asthma. Pharmacoeconomics 2006;24(7):695‐708. - PubMed
-
- Vogelmeier C, D'Urzo A. Maintenance plus as‐needed budesonide/formoterol vs salmeterol/fluticasone in a real‐life setting. European Respiratory Journal 2005;26(Suppl 49):Abstract No. 2770.
-
- Vogelmeier C, D'Urzo A, Jaspal M, Merino JM, Johansson G, Boulet S. Symbicort for both maintenance and relief reduces exacerbations compared with a titration of Seretide (Advair) in patients with asthma: a real life study. American Thoracic Society 2005 International Conference; May 20‐25; San Diego, California. 2005:Poster F67.
D5890C00003 {published data only}
-
- AstraZeneca (D5890C00003). A comparison of the control of asthma inflammation provided by Symbicort Turbuhaler 160/4.5 mcg/inhalation bid plus as‐needed versus symbicort turbuhaler 320/9 mcg/inhalation bid plus Pulmicort Turbuhaler 400mcg/dose bid plus terbutaline turbuhaler 0.4mg/inhalation as‐needed. www.clinicaltrials.gov 2006:http://www.clinicaltrials.gov/ct/show/NCT00244608 [Accessed22/02/2008].
Ind 2002 {published data only}
-
- Ind PW, Villasante C, Shiner RJ, Pietinalho A, Boszormenyi NG, Soliman S, et al. Safety of formoterol by Turbuhaler as reliever medication compared with terbutaline in moderate asthma. European Respiratory Journal 2002;20(4):859‐66. - PubMed
Jenkins 2007 {published data only}
-
- Jenkins CR, Marks GB, Gibson PG, Wark PAB, Thien FC, Belousova EG, et al. A randomised controlled trial of two algorithms for maintaining asthma control on long‐acting bronchodilators (LABA) and inhaled corticosteroids (ICS). Thoracic Society of Australia and New Zealand Annual Scientific Meeting, 25‐28 March 2007, Auckland. 2007:Abstract TP044.
Jonkers 2006 {published data only}
Loukides 2005 {published data only}
-
- Loukides S, Papageorgiou M, Karokis A, Zervas E, Christodoulopoulou A, Papageorgiou N, et al. Single inhaler therapy (SiT) with budesonide/formoterol (BUD/FUM) is effective in asthma control. European Respiratory Journal. 2005; Vol. 26(Suppl 49):124S.
Lundborg 2006 {published data only}
-
- Astrazeneca (LD‐039‐0003). An open, randomized, parallel‐group, multicentre, phase IIIB study to evaluate the efficacy of Symbicort® Turbuhaler® single inhaler therapy (SiT), given as a low maintenance dose once or twice daily plus as needed, compared to a higher maintenance dose of Symbicort Turbuhaler given twice daily plus Oxis® Turbuhaler® as needed during 24 weeks in asthmatic patients (LD‐039‐0003). AstraZeneca Clinical Trials Register 2006, issue http://www.astrazenecaclinicaltrials.com/ncmprint.aspx?type=article&... (accessed 21st February 2008).
-
- Lundborg M, Wille S, Bjermer L, Tilling B, Lundgren M, Telg G, et al. Maintenance plus reliever budesonide/formoterol compared with a higher maintenance dose of budesonide/formoterol plus formoterol as reliever in asthma: An efficacy and cost‐effectiveness study. Current Medical Research and Opinion 2006;22(5):809‐21. - PubMed
NCT00463866 {published data only}
-
- Aubier M, Buhl R, Ekstrom T, Ostinelli J, Schayck CP, Selroos O, et al. Comparison of two twice‐daily doses of budesonide/formoterol maintenance and reliever therapy. European Respiratory Journal 2010; Vol. 36, issue 3:524‐30. - PubMed
Richter 2007 {published data only}
-
- Richter K, Hartmann U, Metzenauer P, Magnussen H. Randomised trial comparing as‐needed versus regular treatment with formoterol in patients with persistent asthma. Respiratory Medicine 2007;101(3):467‐75. - PubMed
SMILE {published and unpublished data}
-
- Rabe F, Atienza T, Magyar P, Larsoon P, Jorup C, Lalloo G. A new combination therapeutic approach challenging the current dogma of using inhaled corticosteroids as maintenance only to control asthma. European Respiratory Journal 2006;28(Suppl 50):666s.
-
- Rabe KF, Atienza T, Magyar P, Larsson P, Jorup C, Lalloo UG. Effect of budesonide in combination with formoterol for reliever therapy in asthma exacerbations: a randomised controlled, double‐blind study. Lancet 2006;938(9537):744‐53. - PubMed
SOMA {published and unpublished data}
-
- Haahtela T, Tamminen K, Malmberg LP, Zetterstrom O, Karjalainen J, Yla‐Outinen H, et al. Formoterol as needed with or without budesonide in patients with intermittent asthma and raised NO levels in exhaled air: A SOMA study. European Respiratory Journal 2006;28(4):748‐55. - PubMed
-
- Haahtela T, Tamminen K, Malmberg P, Karjalainen J, Yia‐Outinen H, Zetterstrom O, et al. As‐needed treatment with a b2‐agonist/ corticosteroid combination in mild intermittent asthma (SOMA). European Respiratory Journal 2005;26(Suppl 45):Abstract No. 1722.
Tattersfield 2001 {published data only}
-
- Berggren F, Ekström T. A cost‐effectiveness study comparing the as‐needed use of formoterol (Oxis) and terbutaline (Bricanyl) in patients with moderate to severe asthma. Respiratory Medicine 2001;98(9):753‐8. - PubMed
-
- Tattersfield AE, Löfdahl CG, Postma DS, Eivindson A, Schreurs AGM, Rasidakis A, et al. Comparison of formoterol and terbutaline for as‐needed treatment of asthma: a randomised trial. Lancet 2001;357(9252):257‐61. - PubMed
Additional references
Adams 2008
Barnes 2007
Bisgaard 2003
-
- Bisgaard H. Effect of long‐acting Beta2 agonists on exacerbation rates of asthma in children. Pediatric Pulmonology 2003; Vol. 36, issue 5:391‐8. [1099‐0496] - PubMed
Cates 2008
Cates 2009a
Cates 2009b
Cates 2010a
-
- Cates CJ, Lasserson TJ. Regular treatment with formoterol and an inhaled corticosteroid versus regular treatment with salmeterol and an inhaled corticosteroid for chronic asthma: serious adverse events. Cochrane Database of Systematic Reviews 2010, Issue 1. [DOI: 10.1002/14651858.CD007694.pub2; CD007694] - DOI - PMC - PubMed
Cates 2011
-
- Cates CJ, Lasserson TJ. Combination formoterol and inhaled steroid as maintenance and reliever therapy versus higher dose combination inhaler maintenance for chronic asthma in adults and children. Cochrane Database of Systematic Reviews 2011, Issue 3. [DOI: 10.1002/14651858.CD009019] - DOI - PMC - PubMed
Cates 2012a
Cates 2012b
Delea 2008
-
- Delea TE, Hagiwara M, Stanford RH, Stempel DA. Effects of fluticasone propionate/salmeterol combination on asthma‐related health care resource utilization and costs and adherence in children and adults with asthma. Clinical Therapeutics 2008; Vol. 30, issue 3:560‐71. - PubMed
Demoly 2009
-
- Demoly P, Louis R, Soes‐Petersen U, Naya I, Carlsheimer A, Worth H, et al. Budesonide/formoterol maintenance and reliever therapy versus conventional best practice. Respiratory Medicine 2009;103(11):1623‐32. - PubMed
Ducharme 2010
-
- Ducharme FM, Ni Chroinin M, Greenstone I, Lasserson TJ. Addition of long‐acting beta2‐agonists to inhaled steroids versus higher dose inhaled steroids in adults and children with persistent asthma. Cochrane Database of Systematic Reviews 2010, Issue 4. [DOI: 10.1002/14651858.CD005533.pub2] - DOI - PMC - PubMed
Ducharme 2011
FitzGerald 2004
GINA 2006
-
- From the Global Strategy for Asthma Management and Prevention, Global Initiative for Asthma (GINA) 2006. http://www.ginasthma.org (accessed 2006).
Harrison 2004
-
- Harrison T, Oborne J, Newton S, Tattersfield A. Doubling the dose of inhaled corticosteroid to prevent asthma exacerbations: randomised controlled trial. Lancet 2004; Vol. 363, issue 9405:271‐5. - PubMed
Higgins 2003
Higgins 2008
-
- Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.0.0 [updated February 2008] Available from: www.cochrane‐handbook.org. The Cochrane Collaboration, 2008.
Lasserson 2008
Lipworth 2008
-
- Lipworth BJ, Jackson C. A SMART choice for primary care asthma therapy?. http://www.bmj.com/cgi/eletters/335/7618/513#178078 13 October 2007.
Ni Chroinin 2005
-
- Ni Chroinin M, Greenstone IR, Danish A, Magdolinos H, Masse V, Zhang X, et al. Long‐acting beta2‐agonists versus placebo in addition to inhaled corticosteroids in children and adults with chronic asthma. Cochrane Database of Systematic Reviews 2005, Issue 4. [DOI: 10.1002/14651858.CD005535] - DOI - PubMed
Ni Chroinin 2009
Palmqvist 2001
-
- Palmqvist M, Arvidsson P, Beckman O, Peterson S, Lotvall J. Onset of bronchodilation of budesonide/formoterol vs. salmeterol/fluticasone in single inhalers. Pulmonary Pharmacology and Therapeutics 2001;14(1):29‐34. - PubMed
SIGN/BTS 2012
-
- Scottish Intercollegiate Guidelines Network/British Thoracic Society. British guideline on the management of asthma: a national clinical guideline. Edinburgh: Scottish Intercollegiate Guidelines Network, 2008 (revised 2012).
Tattersfield 1999
-
- Tattersfield AE, Postma DS, Barnes PJ, Svensson K, Bauer CA, O'Byrne PM, et al. Exacerbations of asthma: a descriptive study of 425 severe exacerbations. The FACET International Study Group. American Journal of Respiratory and Critical Care Medicine 1999;160(2):594‐9. - PubMed
Visual Rx
-
- Visual Rx. www.nntonline.net/visualrx/ Accessed June 2012.
Walters 2007
-
- Walters EH, Gibson PG, Lasserson TJ, Walters JAE. Long‐acting beta2‐agonists for chronic asthma in adults and children where background therapy contains varied or no inhaled corticosteroid. Cochrane Database of Systematic Reviews 2007, Issue 1. [DOI: 10.1002/14651858.CD001385.pub2] - DOI - PMC - PubMed
References to other published versions of this review
Cates 2009
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

