Prime-boost interval matters: a randomized phase 1 study to identify the minimum interval necessary to observe the H5 DNA influenza vaccine priming effect
- PMID: 23633407
- PMCID: PMC3699006
- DOI: 10.1093/infdis/jit180
Prime-boost interval matters: a randomized phase 1 study to identify the minimum interval necessary to observe the H5 DNA influenza vaccine priming effect
Abstract
Background: H5 DNA priming was previously shown to improve the antibody response to influenza A(H5N1) monovalent inactivated vaccine (MIV) among individuals for whom there was a 24-week interval between prime and boost receipt. This study defines the shortest prime-boost interval associated with an improved response to MIV.
Methods: We administered H5 DNA followed by MIV at intervals of 4, 8, 12, 16, or 24 weeks and compared responses to that of 2 doses of MIV (prime-boost interval, 24 weeks).
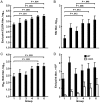
Results: H5 DNA priming with an MIV boost ≥12 weeks later showed an improved response, with a positive hemagglutination inhibition (HAI) titer in 91% of recipients (geometric mean titer [GMT], 141-206), compared with 55%-70% of recipients with an H5 DNA and MIV prime-boost interval of ≤8 weeks (GMT, 51-70) and 44% with an MIV-MIV prime-boost interval of 24 weeks (GMT, 27).
Conclusion: H5 DNA priming enhances antibody responses after an MIV boost when the prime-boost interval is 12-24 weeks. Clinical Trials Registration. NCT01086657.
Keywords: Avian influenza; DNA vaccine; H5N1; boost interval; hemagglutination inhibition.
Figures
References
-
- Treanor JJ, Campbell JD, Zangwill KM, Rowe T, Wolff M. Safety and immunogenicity of an inactivated subvirion influenza A (H5N1) vaccine. N Engl J Med. 2006;354:1343–51. - PubMed
-
- WHO. Confirmed human cases of avian influenza A(H5N1) http://www.who.int/csr/disease/avian_influenza/country/cases_table_2011_.... Accessed 14 August 2011.
-
- Enserink M, Malakoff D. Biosecurity. Will flu papers lead to new research oversight? Science. 2012;335:20–22. –. - PubMed
-
- Kawaoka Y. H5N1: Flu transmission work is urgent. Nature. 2012;482:155. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

