Regulation of FANCD2 by the mTOR pathway contributes to the resistance of cancer cells to DNA double-strand breaks
- PMID: 23633493
- PMCID: PMC3674187
- DOI: 10.1158/0008-5472.CAN-12-4282
Regulation of FANCD2 by the mTOR pathway contributes to the resistance of cancer cells to DNA double-strand breaks
Abstract
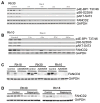
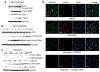
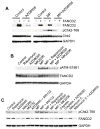
Deregulation of the mTOR pathway is closely associated with tumorigenesis. Accordingly, mTOR inhibitors such as rapamycin and mTOR-selective kinase inhibitors have been tested as cancer therapeutic agents. Inhibition of mTOR results in sensitization to DNA-damaging agents; however, the molecular mechanism is not well understood. We found that an mTOR-selective kinase inhibitor, AZD8055, significantly enhanced sensitivity of a pediatric rhabdomyosarcoma xenograft to radiotherapy and sensitized rhabdomyosarcoma cells to the DNA interstrand cross-linker (ICL) melphalan. Sensitization correlated with drug-induced downregulation of a key component of the Fanconi anemia pathway, FANCD2 through mTOR regulation of FANCD2 gene transcripts via mTORC1-S6K1. Importantly, we show that FANCD2 is required for the proper activation of ATM-Chk2 checkpoint in response to ICL and that mTOR signaling promotes ICL-induced ATM-Chk2 checkpoint activation by sustaining FANCD2. In FANCD2-deficient lymphoblasts, FANCD2 is essential to suppress endogenous and induced DNA damage, and FANCD2-deficient cells showed impaired ATM-Chk2 and ATR-Chk1 activation, which was rescued by reintroduction of wild-type FANCD2. Pharmacologic inhibition of PI3K-mTOR-AKT pathway in Rh30 rhabdomyosarcoma cells attenuated ICL-induced activation of ATM, accompanied with the decrease of FANCD2. These data suggest that the mTOR pathway may promote the repair of DNA double-strand breaks by sustaining FANCD2 and provide a novel mechanism of how the Fanconi anemia pathway modulates DNA damage response and repair.
©2013 AACR.
Conflict of interest statement
Conflict of Interest Statement: The authors consider that there are no actual or perceived competing financial interests.
Figures



References
-
- Shaw RJ, Cantley LC. Ras, PI(3)K and mTOR signalling controls tumour cell growth. Nature. 2006;441:424–30. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

