Effects of cryoprotectants on the structure and thermostability of the human carbonic anhydrase II-acetazolamide complex
- PMID: 23633596
- PMCID: PMC3640473
- DOI: 10.1107/S0907444913002771
Effects of cryoprotectants on the structure and thermostability of the human carbonic anhydrase II-acetazolamide complex
Abstract
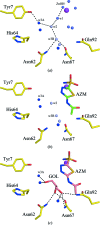
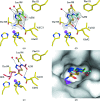
Protein X-ray crystallography has seen a progressive shift from data collection at cool/room temperature (277-298 K) to data collection at cryotemperature (100 K) because of its ease of crystal preparation and the lessening of the detrimental effects of radiation-induced crystal damage, with 20-25%(v/v) glycerol (GOL) being the preferred choice of cryoprotectant. Here, a case study of the effects of cryoprotectants on the kinetics of carbonic anhydrase II (CA II) and its inhibition by the clinically used inhibitor acetazolamide (AZM) is presented. Comparative studies of crystal structure, kinetics, inhibition and thermostability were performed on CA II and its complex with AZM in the presence of either GOL or sucrose. These results suggest that even though the cryoprotectant GOL was previously shown to be directly bound in the active site and to interact with AZM, it affects neither the thermostability of CA II nor the binding of AZM in the crystal structure or in solution. However, addition of GOL does affect the kinetics of CA II, presumably as it displaces the water proton-transfer network in the active site.
Keywords: acetazolamide; carbonic anhydrase; cryoprotectants; glycerol; sucrose.
Figures
References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

