Infectious particles, stress, and induced prion amyloids: a unifying perspective
- PMID: 23633671
- PMCID: PMC3714129
- DOI: 10.4161/viru.24838
Infectious particles, stress, and induced prion amyloids: a unifying perspective
Abstract
Transmissible encephalopathies (TSEs) are believed by many to arise by spontaneous conversion of host prion protein (PrP) into an infectious amyloid (PrP-res, PrP (Sc) ) without nucleic acid. Many TSE agents reside in the environment, with infection controlled by public health measures. These include the disappearance of kuru with the cessation of ritual cannibalism, the dramatic reduction of epidemic bovine encephalopathy (BSE) by removal of contaminated feed, and the lack of endemic scrapie in geographically isolated Australian sheep with susceptible PrP genotypes. While prion protein modeling has engendered an intense focus on common types of protein misfolding and amyloid formation in diverse organisms and diseases, the biological characteristics of infectious TSE agents, and their recognition by the host as foreign entities, raises several fundamental new directions for fruitful investigation such as: (1) unrecognized microbial agents in the environmental metagenome that may cause latent neurodegenerative disease, (2) the evolutionary social and protective functions of different amyloid proteins in diverse organisms from bacteria to mammals, and (3) amyloid formation as a beneficial innate immune response to stress (infectious and non-infectious). This innate process however, once initiated, can become unstoppable in accelerated neuronal aging.
Keywords: Alzheimer disease; Parkinson disease; aging; biofilms; environmental pathogens; latency; metagenome; nucleic acids; transmissible encephalopathies; yeast prions.
Figures

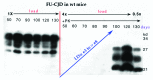

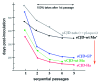
References
-
- Chiesa R, Drisaldi B, Quaglio E, Migheli A, Piccardo P, Ghetti B, et al. Accumulation of protease-resistant prion protein (PrP) and apoptosis of cerebellar granule cells in transgenic mice expressing a PrP insertional mutation. Proc Natl Acad Sci U S A. 2000;97:5574–9. doi: 10.1073/pnas.97.10.5574. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
