Rapid in vivo assessment of drug efficacy against Mycobacterium tuberculosis using an improved firefly luciferase
- PMID: 23633686
- PMCID: PMC3743513
- DOI: 10.1093/jac/dkt155
Rapid in vivo assessment of drug efficacy against Mycobacterium tuberculosis using an improved firefly luciferase
Abstract
Objectives: In vivo experimentation is costly and time-consuming, and presents a major bottleneck in anti-tuberculosis drug development. Conventional methods rely on the enumeration of bacterial colonies, and it can take up to 4 weeks for Mycobacterium tuberculosis to grow on agar plates. Light produced by recombinant bacteria expressing luciferase enzymes can be used as a marker of bacterial load, and disease progression can be easily followed non-invasively in live animals by using the appropriate imaging equipment. The objective of this work was to develop a bioluminescence-based mouse model of tuberculosis to assess antibiotic efficacy against M. tuberculosis in vivo.
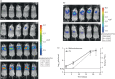
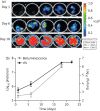
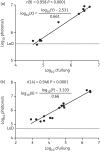
Methods: We used an M. tuberculosis strain carrying a red-shifted derivative of the firefly luciferase gene (FFlucRT) to infect mice, and monitored disease progression in living animals by bioluminescence imaging before and after treatment with the frontline anti-tuberculosis drug isoniazid. The resulting images were analysed and the bioluminescence was correlated with bacterial counts.
Results: Using bioluminescence imaging we detected as few as 1.7 × 10(3) and 7.5 × 10(4) reporter bacteria ex vivo and in vivo, respectively, in the lungs of mice. A good correlation was found between bioluminescence and bacterial load in both cases. Furthermore, a marked reduction in luminescence was observed in living mice given isoniazid treatment.
Conclusions: We have shown that an improved bioluminescent strain of M. tuberculosis can be visualized by non-invasive imaging in live mice during an acute, progressive infection and that this technique can be used to rapidly visualize and quantify the effect of antibiotic treatment. We believe that the model presented here will be of great benefit in early drug discovery as an easy and rapid way to identify active compounds in vivo.
Keywords: bioluminescence; drug testing; mouse model; optical imaging.
Figures




References
-
- WHO. Tuberculosis Fact Sheet. http://www.who.int/mediacentre/factsheets/fs104/en/index.html. (10 April 2013, date last accessed)
-
- Rice BW, Cable MD, Nelson MB. In vivo imaging of light-emitting probes. J Biomed Opt. 2001;6:432–40. - PubMed
-
- Zhao H, Doyle TC, Coquoz O, et al. Emission spectra of bioluminescent reporters and interaction with mammalian tissue determine the sensitivity of detection in vivo. J Biomed Opt. 2005;10:41210. - PubMed
-
- Prioli RP, Tanna A, Brown IN. Rapid methods for counting mycobacteria–comparison of methods for extraction of mycobacterial adenosine triphosphate (ATP) determined by firefly luciferase assay. Tubercle. 1985;66:99–108. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

