Knowledge, attitudes, and practice of doctors to adverse drug reaction reporting in a teaching hospital in India: An observational study
- PMID: 23633861
- PMCID: PMC3633276
- DOI: 10.4103/0976-9668.107289
Knowledge, attitudes, and practice of doctors to adverse drug reaction reporting in a teaching hospital in India: An observational study
Abstract
Background: Underreporting of spontaneous adverse drug reaction (ADR) is a threat to pharmacovigilance. Various factors related with the knowledge and attitudes are responsible for underreporting of ADRs.
Aims: The study was aimed at investigating the knowledge and attitudes of doctors to ADR reporting.
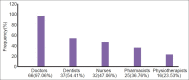
Materials and methods: It was a questionnaire-based cross-sectional study. One hundred and eight questionnaires were administered to doctors working in a teaching hospital with an ADR monitoring center.
Statistical analysis used: The descriptive statistics were used for responses to evaluate the knowledge and attitudes toward ADR reporting. Pearson's Chi-square test was used to observe the association of knowledge and attitude with experience and position.
Results: The response rate was 62.9%. Spontaneous reporting rate was found to be 19.1%. The major factors found to be responsible for underreporting of ADR include inadequate risk perception about newly marketed drugs (77.9%), fear factor (73.5%), diffidence (67.7%), lack of clarity of information on ADR form about reporting (52.9%), lethargy (42.7%), insufficient training to identify ADRs (41.2%), lack of awareness about existence of pharmacovigilance program (30.9%) and ADR monitoring center in the institute (19.1%), and inadequate risk perception of over-the-counter (OTC) product (20.6%) and herbal medicines (13.2%). Experience and position did not influence the knowledge and attitudes of doctors.
Conclusion: The deficiencies in knowledge and attitudes require urgent attention not only to improve the rate of spontaneous reporting, but also for enhanced safety of the patients and society at large.
Keywords: Attitude; doctors; knowledge; pharmacovigilance; spontaneous reporting.
Conflict of interest statement
Figures
References
-
- International drug monitoring: The role of National Centres. Report No: 498. Geneva, Switzerland: World Health Organization; 1972. World Health Organization. - PubMed
-
- Lazarou J, Pomeranz BH, Corey PN. Incidence of adverse drug reactions in hospitalized patients: A meta-analysis of prospective studies. JAMA. 1998;279:1200–5. - PubMed
-
- The importance of pharmacovigilance. Safety monitoring of medicinal products. Geneva: World Health Organization; 2002. World Health Organization Collaborating Centre for International Drug Monitoring.
-
- Pharmacovigilance programme of India 2010. CDSCO, Ministry of Health and Family Welfare, Government of India. 2010. [Last accessed on 2012 March 21]. Available from: http://cdsco.nic.in/pharmacovigilance.htm .
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials