(1'S,12'R,13'S,17'S)-15',15'-Dimethyl-1,2-dihydro-11',14',16',18'-tetra-oxa-7'-aza-spiro-[indole-3,8'-penta-cyclo-[10.6.0.0(2,9).0(3,7).0(13,17)]octa-deca-ne]-2,10'-dione
- PMID: 23634025
- PMCID: PMC3629507
- DOI: 10.1107/S1600536813005436
(1'S,12'R,13'S,17'S)-15',15'-Dimethyl-1,2-dihydro-11',14',16',18'-tetra-oxa-7'-aza-spiro-[indole-3,8'-penta-cyclo-[10.6.0.0(2,9).0(3,7).0(13,17)]octa-deca-ne]-2,10'-dione
Abstract
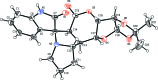
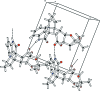
In the title compound, C22H24N2O6, the indole ring has a twist conformation and the tetra-hydro-2H-pyran-2-one ring a half-chair conformation. One of the pyrrolidine rings adopts an envelope conformation on the N atom, while the other has a twist conformation; the 'butterfly' angle between their mean planes is 62.98 (11)°. The dioxolane ring adopts a twist conformation and the tetra-hydro-furan ring has an envelope conformation on the C atom in the fused tetra-hydro-2H-pyran-2-one ring adjacent to the O atom of the tetra-hydro-furan ring. The 'butterfly' angle between the mean planes of these two five-membered rings is 69.14 (10)°. In the crystal, mol-ecules are linked by N-H⋯O hydrogen bonds, forming chains along the a axis.
Figures
References
-
- Amal Raj, A., Raghunathan, R., SrideviKumari, M. R. & Raman, N. (2003). Bioorg. Med. Chem. 11, 407–419. - PubMed
-
- Bruker (2008). APEX2, SAINT and SADABS Bruker AXS Inc., Madison Wisconsin, USA.
-
- Farrugia, L. J. (2012). J. Appl. Cryst. 45, 849–854.
-
- Rajeswaran, W. G., Labroo, R. B., Cohen, L. A. & King, M. M. (1999). J. Org. Chem. 64, 1369–1371.
LinkOut - more resources
Full Text Sources
Other Literature Sources
