3-Amino-1-(4-fluoro-phen-yl)-7-meth-oxy-1H-benzo[f]chromene-2-carbonitrile
- PMID: 23634034
- PMCID: PMC3629516
- DOI: 10.1107/S1600536813005473
3-Amino-1-(4-fluoro-phen-yl)-7-meth-oxy-1H-benzo[f]chromene-2-carbonitrile
Abstract
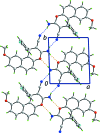
In the title compound, C21H15FN2O2, the furan ring has a flattened half-chair conformation [the methine C atom lies 0.136 (2) Å above the C5 plane which has an r.m.s. deviation of 0.0229 Å]. Overall, the 1H-benzo[f]chromene fused-ring system approximates a plane (r.m.s. deviation of the 14 non-H atoms = 0.049 Å). The fluoro-benzene ring is almost perpendicular to this plane [dihedral angle = 89.58 (8)°]. Zigzag supra-molecular tapes along the b axis are the most notable feature of the crystal packing. This arises through an alternating sequence of 12-membered {⋯HNC3N}2 and eight-membered {⋯HNCO}2 synthons. These are connected into a three-dimensional architecture by π-π [inter-centroid distance for centrosymmetrically related fluoro-benzene rings = 3.5181 (10) Å] and C-H⋯π inter-actions.
Figures


References
-
- Agilent (2011). CrysAlis PRO Agilent Technologies, Yarnton, England.
-
- Brandenburg, K. (2006). DIAMOND Crystal Impact GbR, Bonn, Germany.
-
- Farrugia, L. J. (2012). J. Appl. Cryst. 45, 849–854.
-
- Shekhar, A. C., Kumar, A. R., Sathaiah, G., Raju, K., Rao, P. S., Sridhar, M., Narsaiah, B., Srinivas, P. V. S. S. & Sridhar, B. (2012). Helv. Chim. Acta, 95, 502–508.
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous
