Dimethyl 1-(4-methyl-phen-yl)-8-(thio-phen-2-yl)-11-oxatricyclo-[6.2.1.0(2,7)]undeca-2,4,6,9-tetra-ene-9,10-dicarboxy-late
- PMID: 23634047
- PMCID: PMC3629529
- DOI: 10.1107/S1600536813005308
Dimethyl 1-(4-methyl-phen-yl)-8-(thio-phen-2-yl)-11-oxatricyclo-[6.2.1.0(2,7)]undeca-2,4,6,9-tetra-ene-9,10-dicarboxy-late
Abstract
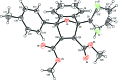
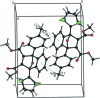
The title compound, C25H20O5S, is the product of a Diels-Alder reaction. The mol-ecule consists of a fused tricyclic system containing two five-membered rings and one six-membered ring. The five-membered rings both show an envelope conformation with the O atom at the flap, whereas the six-membered ring adopts a boat conformation. The thio-phene ring is disordered over two sets of sites with an occupancy ratio of 0.53 (1):0.47 (1). The dihedral angles between the 4-methyl-phenyl ring and the major and minor components of the thio-phene ring are 66.3 (1) and 67.9 (1)°, respectively, while the dihedral angle between the disordered thio-phenyl components is 3.1 (1)°. The mean plane of the tricyclic ring system makes dihedral angles of 35.8 (1), 30.8 (1) and 32.8 (1)°, respectively, with the 4-methyl-phenyl ring and the major and minor components of the thio-phenyl ring. In the crystal, inversion dimers are formed through pairs of C-H⋯π inter-actions. In addition, C-H⋯O inter-actions are observed.
Figures
References
-
- Bruker (2008). APEX2 and SAINT Bruker AXS Inc., Madison. Wisconsin, USA.
-
- Cremer, D. & Pople, J. A. (1975). J. Am. Chem. Soc. 97, 1354–1358.
-
- Denmark, S. E. & Thorarensen, A. (1996). Chem. Rev. 96, 137–166. - PubMed
-
- Farrugia, L. J. (2012). J. Appl. Cryst. 45, 849–854.
LinkOut - more resources
Full Text Sources
Other Literature Sources