Amicarbazone
- PMID: 23634130
- PMCID: PMC3629643
- DOI: 10.1107/S1600536813007782
Amicarbazone
Abstract
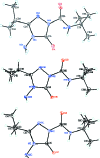
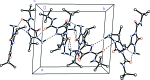
Three independent mol-ecules comprise the asymmetric unit of the title compound, C10H19N5O2, (systematic name: 4-amino-N-tert-butyl-3-isopropyl-5-oxo-4,5-dihydro-1H-1,2,4-triazole-1-carboxamide) . In all three mol-ecules, the triazole ring and the carboxamide group are almost coplanar [within 4.0-5.9 (9)°], particularly because of the formation of an intra-molecular N-H⋯O hydrogen bond. On other hand, the orientation of the isopropyl group varies significantly from mol-ecule to mol-ecule. The crystal packing is dominated by N-H⋯O and N-H⋯N hydrogen bonds, which connect the mol-ecules into infinite chains along [010].
Figures
References
-
- Agilent (2012). CrysAlis PRO and CrysAlis RED Agilent Technologies, Yarnton, England.
-
- Allen, F. H., Kennard, O., Watson, D. G., Brammer, L., Orpen, A. G. & Taylor, R. (1987). J. Chem. Soc. Perkin Trans. 2, pp. S1–19.
-
- Crockett, R., Forrester, A. R. & Howie, R. A. (2004). Acta Cryst. E60, o460–o461.
-
- Dayan, F. E., Trindade, M. L. B. & Velini, E. D. (2009). Weed Sci. 57, 579–583.
-
- Diehr (1998). US Patent No. US005708184A.
LinkOut - more resources
Full Text Sources
Other Literature Sources
