Inhibition of nuclear factor-kappa B activation decreases survival of Mycobacterium tuberculosis in human macrophages
- PMID: 23634218
- PMCID: PMC3636238
- DOI: 10.1371/journal.pone.0061925
Inhibition of nuclear factor-kappa B activation decreases survival of Mycobacterium tuberculosis in human macrophages
Abstract
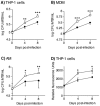
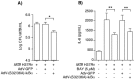
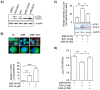
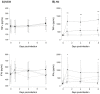
Nuclear factor-kappa B (NFκB) is a ubiquitous transcription factor that mediates pro-inflammatory responses required for host control of many microbial pathogens; on the other hand, NFκB has been implicated in the pathogenesis of other inflammatory and infectious diseases. Mice with genetic disruption of the p50 subunit of NFκB are more likely to succumb to Mycobacterium tuberculosis (MTB). However, the role of NFκB in host defense in humans is not fully understood. We sought to examine the role of NFκB activation in the immune response of human macrophages to MTB. Targeted pharmacologic inhibition of NFκB activation using BAY 11-7082 (BAY, an inhibitor of IκBα kinase) or an adenovirus construct with a dominant-negative IκBα significantly decreased the number of viable intracellular mycobacteria recovered from THP-1 macrophages four and eight days after infection. The results with BAY were confirmed in primary human monocyte-derived macrophages and alveolar macrophages. NFκB inhibition was associated with increased macrophage apoptosis and autophagy, which are well-established killing mechanisms of intracellular MTB. Inhibition of the executioner protease caspase-3 or of the autophagic pathway significantly abrogated the effects of BAY. We conclude that NFκB inhibition decreases viability of intracellular MTB in human macrophages via induction of apoptosis and autophagy.
Conflict of interest statement
Figures



Similar articles
-
Endoplasmic reticulum stress pathway-mediated apoptosis in macrophages contributes to the survival of Mycobacterium tuberculosis.PLoS One. 2011;6(12):e28531. doi: 10.1371/journal.pone.0028531. Epub 2011 Dec 14. PLoS One. 2011. PMID: 22194844 Free PMC article.
-
Curcumin enhances human macrophage control of Mycobacterium tuberculosis infection.Respirology. 2016 Jul;21(5):951-7. doi: 10.1111/resp.12762. Epub 2016 Mar 24. Respirology. 2016. PMID: 27012592
-
Phenylbutyrate induces LL-37-dependent autophagy and intracellular killing of Mycobacterium tuberculosis in human macrophages.Autophagy. 2015;11(9):1688-99. doi: 10.1080/15548627.2015.1075110. Autophagy. 2015. PMID: 26218841 Free PMC article.
-
Shedding Light on Autophagy During Human Tuberculosis. A Long Way to Go.Front Cell Infect Microbiol. 2022 Jan 5;11:820095. doi: 10.3389/fcimb.2021.820095. eCollection 2021. Front Cell Infect Microbiol. 2022. PMID: 35071056 Free PMC article. Review.
-
Potential roles of the NFκB and glutathione pathways in mature human erythrocytes.Cell Mol Biol Lett. 2012 Mar;17(1):11-20. doi: 10.2478/s11658-011-0032-x. Epub 2011 Nov 21. Cell Mol Biol Lett. 2012. PMID: 22105338 Free PMC article. Review.
Cited by
-
Autophagy in Mycobacterium tuberculosis and HIV infections.Front Cell Infect Microbiol. 2015 Jun 2;5:49. doi: 10.3389/fcimb.2015.00049. eCollection 2015. Front Cell Infect Microbiol. 2015. PMID: 26082897 Free PMC article. Review.
-
Antibiotic adjuvants: synergistic tool to combat multi-drug resistant pathogens.Front Cell Infect Microbiol. 2023 Dec 20;13:1293633. doi: 10.3389/fcimb.2023.1293633. eCollection 2023. Front Cell Infect Microbiol. 2023. PMID: 38179424 Free PMC article. Review.
-
BCG vaccination reduces the mortality of Mycobacterium tuberculosis-infected type 2 diabetes mellitus mice.JCI Insight. 2020 Mar 12;5(5):e133788. doi: 10.1172/jci.insight.133788. JCI Insight. 2020. PMID: 32161191 Free PMC article.
-
Nontuberculous mycobacterial lung infections in patients with eating disorders: plausible mechanistic links in a case series.AME Case Rep. 2021 Jan 25;5:9. doi: 10.21037/acr-20-101. eCollection 2021. AME Case Rep. 2021. PMID: 33634249 Free PMC article.
-
Guidelines for the use and interpretation of assays for monitoring autophagy (4th edition)1.Autophagy. 2021 Jan;17(1):1-382. doi: 10.1080/15548627.2020.1797280. Epub 2021 Feb 8. Autophagy. 2021. PMID: 33634751 Free PMC article.
References
-
- Chan ED, Iseman MD (2008) Multidrug-resistant and extensively drug-resistant tuberculosis: a review. Curr Opin Infect Dis 21: 587–595. - PubMed
-
- Chiang CY, Yew WW (2009) Multidrug-resistant and extensively drug-resistant tuberculosis. Int J Tuberc Lung Dis 13: 304–311. - PubMed
-
- Madariaga MG, Lalloo UG, Swindells S (2008) Extensively drug-resistant tuberculosis. Am J Med 121: 835–844. - PubMed
-
- Deretic V, Singh S, Master S, Harris J, Roberts E, et al. (2006) Mycobacterium tuberculosis inhibition of phagolysosome biogenesis and autophagy as a host defence mechanism. Cell Microbiol 8: 719–727. - PubMed
-
- Ferrari G, Langen H, Naito M, Pieters J (1999) A coat protein on phagosomes involved in the intracellular survival of mycobacteria. Cell 97: 435–447. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

