Novel insight into the distribution of L-cells in the rat intestinal tract
- PMID: 23634245
- PMCID: PMC3633977
Novel insight into the distribution of L-cells in the rat intestinal tract
Abstract
Background: Gut secreted incretin hormones and gastric bypass surgery currently provides some of the most successful treatments for diabetes and obesity respectively. However, despite the evident importance of the gut endocrine system no information exists on the total number and distribution of different types of endocrine cells in the gut. Here we have used the established preclinical Zucker Diabetic Fatty (ZDF) rat model which displays elevated levels of GLP-1 to assess L-cell distribution and L-cell dynamics in the full rostro-caudal extension of the rat intestinal tract.
Methods: Using mathematically unbiased stereology we provide total and regional estimates of gut volume, gut surface area and the total number of L-cells throughout the intestinal tract in obese ZDF rats and lean controls.
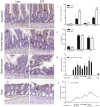
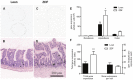
Results: The total number of L-cells in the lean and obese ZDF gut is estimated to 4.8 and 10.9 million, respectively, coupled with a corresponding near doubling in total gut volume and total surface area. L-cell numbers were found to be distributed rather evenly throughout the jejunum, ileum and colon.
Conclusion: The present study provides the first stereological report of total L-cell number and L-cell distribution throughout the rat intestinal tract. In contrast to the currently held view, the majority of L-cells are actually located proximal to the traditionally defined ileum and colon.
Keywords: GLP-1; L-cell; ZDF; diabetes; stereology.
Figures


References
-
- Sjostrom L. Bariatric surgery and reduction in morbidity and mortality: experiences from the SOS study. Int J Obes (Lond) 2008;32(Suppl 7):S93–97. - PubMed
-
- Kim S, Richards WO. Long-term follow-up of the metabolic profiles in obese patients with type 2 diabetes mellitus after Roux-en-Y gastric bypass. Ann Surg. 2010;251:1049–1055. - PubMed
-
- Buchwald H, Estok R, Fahrbach K, Banel D, Jensen MD, Pories WJ, Bantle JP, Sledge I. Weight and type 2 diabetes after bariatric surgery: systematic review and meta-analysis. Am J Med. 2009;122:248–256. 245. - PubMed
-
- Tharakan G, Tan T, Bloom S. Emerging therapies in the treatment of ‘diabesity’: beyond GLP-1. Trends Pharmacol Sci. 2011;32:8–15. - PubMed
LinkOut - more resources
Full Text Sources
