The CDK Network: Linking Cycles of Cell Division and Gene Expression
- PMID: 23634260
- PMCID: PMC3636752
- DOI: 10.1177/1947601912473308
The CDK Network: Linking Cycles of Cell Division and Gene Expression
Abstract
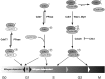
Cyclin-dependent kinases (CDKs) play essential roles in cell proliferation and gene expression. Although distinct sets of CDKs work in cell division and transcription by RNA polymerase II (Pol II), they share a CDK-activating kinase (CAK), which is itself a CDK-Cdk7-in metazoans. Thus a unitary CDK network controls and may coordinate cycles of cell division and gene expression. Recent work reveals decisive roles for Cdk7 in both pathways. The CAK function of Cdk7 helps determine timing of activation and cyclin-binding preferences of different CDKs during the cell cycle. In the transcription cycle, Cdk7 is both an effector kinase, which phosphorylates Pol II and other proteins and helps establish promoter-proximal pausing; and a CAK for Cdk9 (P-TEFb), which releases Pol II from the pause. By governing the transition from initiation to elongation, Cdk7, Cdk9 and their substrates influence expression of genes important for developmental and cell-cycle decisions, and ensure co-transcriptional maturation of Pol II transcripts. Cdk7 engaged in transcription also appears to be regulated by phosphorylation within its own activation (T) loop. Here I review recent studies of CDK regulation in cell division and gene expression, and propose a model whereby mitogenic signals trigger a cascade of CDK T-loop phosphorylation that drives cells past the restriction (R) point, when continued cell-cycle progression becomes growth factor-independent. Because R-point control is frequently deregulated in cancer, the CAK-CDK pathway is an attractive target for chemical inhibition aimed at impeding the inappropriate commitment to cell division.
Keywords: CDK-activating kinase (CAK); RNA polymerase II; cell division cycle; cyclin-dependent kinase (CDK); phosphorylation; transcription.
Conflict of interest statement
Figures
References
-
- Morgan DO. The cell cycle: principles of control. London: New Science Press Ltd; 2007
-
- Yao G, Lee TJ, Mori S, Nevins JR, You L. A bistable Rb-E2F switch underlies the restriction point. Nat Cell Biol. 2008;10(4):476-82 - PubMed
-
- Diffley JF. Regulation of early events in chromosome replication. Curr Biol. 2004;14(18):R778- 86 - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous
