Comparison of a loop-mediated isothermal amplification for orf virus with quantitative real-time PCR
- PMID: 23634981
- PMCID: PMC3651318
- DOI: 10.1186/1743-422X-10-138
Comparison of a loop-mediated isothermal amplification for orf virus with quantitative real-time PCR
Abstract
Background: Orf virus (ORFV) causes orf (also known as contagious ecthyma or contagious papular dermatitis), a severe infectious skin disease in goats, sheep and other ruminants. Therefore, a rapid, highly specific and accurate method for the diagnosis of ORFV infections is essential to ensure that the appropriate treatments are administered and to reduce economic losses.
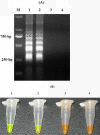
Methods: A loop-mediated isothermal amplification (LAMP) assay based on the identification of the F1L gene was developed for the specific detection of ORFV infections. The sensitivity and specificity of the LAMP assay were evaluated, and the effectiveness of this method was compared with that of real-time PCR.
Results: The sensitivity of this assay was determined to be 10 copies of a standard plasmid. Furthermore, no cross-reactivity was found with either capripox virus or FMDV. The LAMP and real-time PCR assays were both able to detect intracutaneous- and cohabitation-infection samples, with a concordance of 97.83%. LAMP demonstrated a sensitivity of 89.13%.
Conclusion: The LAMP assay is a highly efficient and practical method for detecting ORFV infection. This LAMP method shows great potential for monitoring the prevalence of orf, and it could prove to be a powerful supplemental tool for current diagnostic methods.
Figures



Similar articles
-
Development of a fluorescent probe-based recombinase polymerase amplification assay for rapid detection of Orf virus.Virol J. 2015 Dec 2;12:206. doi: 10.1186/s12985-015-0440-z. Virol J. 2015. PMID: 26631157 Free PMC article.
-
TaqMan based real-time duplex PCR for simultaneous detection and quantitation of capripox and orf virus genomes in clinical samples.J Virol Methods. 2014 Jun;201:44-50. doi: 10.1016/j.jviromet.2014.02.007. Epub 2014 Feb 16. J Virol Methods. 2014. PMID: 24552953
-
Rapid detection of orf virus by loop-mediated isothermal amplification based on the DNA polymerase gene.Arch Virol. 2013 Apr;158(4):793-8. doi: 10.1007/s00705-012-1526-1. Epub 2012 Nov 27. Arch Virol. 2013. PMID: 23183830
-
Recent advances in diagnostic approaches for orf virus.Appl Microbiol Biotechnol. 2023 Mar;107(5-6):1515-1523. doi: 10.1007/s00253-023-12412-8. Epub 2023 Feb 1. Appl Microbiol Biotechnol. 2023. PMID: 36723701 Review.
-
[Ecthyma of sheep and goats].Tierarztl Prax. 1985;13(2):163-9. Tierarztl Prax. 1985. PMID: 3895568 Review. German.
Cited by
-
Molecular diagnosis of COVID-19 in different biologic matrix, their diagnostic validity and clinical relevance: A systematic review.Life Sci. 2020 Oct 1;258:118207. doi: 10.1016/j.lfs.2020.118207. Epub 2020 Aug 7. Life Sci. 2020. PMID: 32777301 Free PMC article.
-
A novel approach for evaluating the performance of real time quantitative loop-mediated isothermal amplification-based methods.Biomol Detect Quantif. 2014 Nov 22;2:4-10. doi: 10.1016/j.bdq.2014.11.001. eCollection 2014 Dec. Biomol Detect Quantif. 2014. PMID: 27896139 Free PMC article.
-
Rapid detection of contagious ecthyma by loop-mediated isothermal amplification and epidemiology in Jilin Province China.J Vet Med Sci. 2016 Jan;78(1):125-8. doi: 10.1292/jvms.15-0340. Epub 2015 Sep 6. J Vet Med Sci. 2016. PMID: 26346652 Free PMC article.
-
An Auto-Reading probe system for detecting deletion mutations In liquid biopsy with direct quantification of mutation abundance.Heliyon. 2024 Jul 31;10(16):e35530. doi: 10.1016/j.heliyon.2024.e35530. eCollection 2024 Aug 30. Heliyon. 2024. PMID: 39220964 Free PMC article.
-
Loop-mediated isothermal amplification for rapid and semiquantitative detection of Loa loa infection.J Clin Microbiol. 2014 Jun;52(6):2071-7. doi: 10.1128/JCM.00525-14. Epub 2014 Apr 2. J Clin Microbiol. 2014. PMID: 24696020 Free PMC article.
References
-
- Haig DM, Mercer AA. Ovine Diseases: Orf. Vet Res. 1998;29:311–326. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
LinkOut - more resources
Full Text Sources
Other Literature Sources

