Maximizing the biological effect of proton dose delivered with scanned beams via inhomogeneous daily dose distributions
- PMID: 23635256
- PMCID: PMC3651218
- DOI: 10.1118/1.4801897
Maximizing the biological effect of proton dose delivered with scanned beams via inhomogeneous daily dose distributions
Abstract
Purpose: Biological effect of radiation can be enhanced with hypofractionation, localized dose escalation, and, in particle therapy, with optimized distribution of linear energy transfer (LET). The authors describe a method to construct inhomogeneous fractional dose (IFD) distributions, and evaluate the potential gain in the therapeutic effect from their delivery in proton therapy delivered by pencil beam scanning.
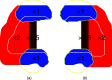
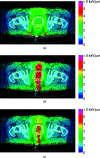
Methods: For 13 cases of prostate cancer, the authors considered hypofractionated courses of 60 Gy delivered in 20 fractions. (All doses denoted in Gy include the proton's mean relative biological effectiveness (RBE) of 1.1.) Two types of plans were optimized using two opposed lateral beams to deliver a uniform dose of 3 Gy per fraction to the target by scanning: (1) in conventional full-target plans (FTP), each beam irradiated the entire gland, (2) in split-target plans (STP), beams irradiated only the respective proximal hemispheres (prostate split sagittally). Inverse planning yielded intensity maps, in which discrete position control points of the scanned beam (spots) were assigned optimized intensity values. FTP plans preferentially required a higher intensity of spots in the distal part of the target, while STP, by design, employed proximal spots. To evaluate the utility of IFD delivery, IFD plans were generated by rearranging the spot intensities from FTP or STP intensity maps, separately as well as combined using a variety of mixing weights. IFD courses were designed so that, in alternating fractions, one of the hemispheres of the prostate would receive a dose boost and the other receive a lower dose, while the total physical dose from the IFD course was roughly uniform across the prostate. IFD plans were normalized so that the equivalent uniform dose (EUD) of rectum and bladder did not increase, compared to the baseline FTP plan, which irradiated the prostate uniformly in every fraction. An EUD-based model was then applied to estimate tumor control probability (TCP) and normal tissue complication probability (NTCP). To assess potential local RBE variations, LET distributions were calculated with Monte Carlo, and compared for different plans. The results were assessed in terms of their sensitivity to uncertainties in model parameters and delivery.
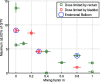
Results: IFD courses included equal number of fractions boosting either hemisphere, thus, the combined physical dose was close to uniform throughout the prostate. However, for the entire course, the prostate EUD in IFD was higher than in conventional FTP by up to 14%, corresponding to the estimated increase in TCP to 96% from 88%. The extent of gain depended on the mixing factor, i.e., relative weights used to combine FTP and STP spot weights. Increased weighting of STP typically yielded a higher target EUD, but also led to increased sensitivity of dose to variations in the proton's range. Rectal and bladder EUD were same or lower (per normalization), and the NTCP for both remained below 1%. The LET distributions in IFD also depended strongly on the mixing weights: plans using higher weight of STP spots yielded higher LET, indicating a potentially higher local RBE.
Conclusions: In proton therapy delivered by pencil beam scanning, improved therapeutic outcome can potentially be expected with delivery of IFD distributions, while administering the prescribed quasi-uniform dose to the target over the entire course. The biological effectiveness of IFD may be further enhanced by optimizing the LET distributions. IFD distributions are characterized by a dose gradient located in proximity of the prostate's midplane, thus, the fidelity of delivery would depend crucially on the precision with which the proton range could be controlled.
Figures




Similar articles
-
SU-E-T-552: Maximizing the Biological Effect of Proton Dose Delivered with Scanned Beam.Med Phys. 2012 Jun;39(6Part18):3832. doi: 10.1118/1.4735641. Med Phys. 2012. PMID: 28518512
-
Inclusion of a variable RBE into proton and photon plan comparison for various fractionation schedules in prostate radiation therapy.Med Phys. 2017 Mar;44(3):810-822. doi: 10.1002/mp.12117. Med Phys. 2017. PMID: 28107554
-
Radiobiological and dosimetric impact of RayStation pencil beam and Monte Carlo algorithms on intensity-modulated proton therapy breast cancer plans.J Appl Clin Med Phys. 2019 Aug;20(8):36-46. doi: 10.1002/acm2.12676. Epub 2019 Jul 25. J Appl Clin Med Phys. 2019. PMID: 31343826 Free PMC article.
-
Proton therapy - Present and future.Adv Drug Deliv Rev. 2017 Jan 15;109:26-44. doi: 10.1016/j.addr.2016.11.006. Epub 2016 Dec 3. Adv Drug Deliv Rev. 2017. PMID: 27919760 Free PMC article. Review.
-
Tumor Control Probability Modeling and Systematic Review of the Literature of Stereotactic Body Radiation Therapy for Prostate Cancer.Int J Radiat Oncol Biol Phys. 2021 May 1;110(1):227-236. doi: 10.1016/j.ijrobp.2020.08.014. Epub 2020 Sep 6. Int J Radiat Oncol Biol Phys. 2021. PMID: 32900561 Free PMC article.
Cited by
-
Simultaneous optimization of dose distributions and fractionation schemes in particle radiotherapy.Med Phys. 2013 Sep;40(9):091702. doi: 10.1118/1.4816658. Med Phys. 2013. PMID: 24007135 Free PMC article.
-
Linear Energy Transfer and Relative Biological Effectiveness Investigation of Various Structures for a Cohort of Proton Patients With Brain Tumors.Adv Radiat Oncol. 2022 Nov 26;8(2):101128. doi: 10.1016/j.adro.2022.101128. eCollection 2023 Mar-Apr. Adv Radiat Oncol. 2022. PMID: 36632089 Free PMC article.
-
A Systematic Review of LET-Guided Treatment Plan Optimisation in Proton Therapy: Identifying the Current State and Future Needs.Cancers (Basel). 2023 Aug 25;15(17):4268. doi: 10.3390/cancers15174268. Cancers (Basel). 2023. PMID: 37686544 Free PMC article. Review.
-
Dosimetric study of uniform scanning proton therapy planning for prostate cancer patients with a metal hip prosthesis, and comparison with volumetric-modulated arc therapy.J Appl Clin Med Phys. 2014 May 8;15(3):4611. doi: 10.1120/jacmp.v15i3.4611. J Appl Clin Med Phys. 2014. PMID: 24892333 Free PMC article.
-
A treatment planning study of urethra-sparing intensity-modulated proton therapy for localized prostate cancer.Phys Imaging Radiat Oncol. 2021 Oct 8;20:23-29. doi: 10.1016/j.phro.2021.09.006. eCollection 2021 Oct. Phys Imaging Radiat Oncol. 2021. PMID: 34693040 Free PMC article.
References
-
- Steneker M., Trofimov A., Hong T., and Engelsman M., “Isotoxic dose escalation by increasing tumor dose variance,” in Proceedings of XVI International Conference on the Use of Computers in Radiation Therapy, edited by van Herk M. and Sonke J. J. (Amsterdam, 2010).
-
- Trofimov A., Nguyen P. L., Lu M. W., Unkelbach J., Kang J., Bortfeld T., and Zietman A. L., “Feasibility of hemi-prostate dose escalation to 91 Gy with 3D-conformal vs intensity-modulated proton therapy,” Int. J. Radiat. Oncol., Biol., Phys. 75, S703–S704 (2009).10.1016/j.ijrobp.2009.07.1604 - DOI
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

