Treatment of malignant effusion by oncolytic virotherapy in an experimental subcutaneous xenograft model of lung cancer
- PMID: 23635329
- PMCID: PMC3646671
- DOI: 10.1186/1479-5876-11-106
Treatment of malignant effusion by oncolytic virotherapy in an experimental subcutaneous xenograft model of lung cancer
Abstract
Background: Malignant pleural effusion (MPE) is associated with advanced stages of lung cancer and is mainly dependent on invasion of the pleura and expression of vascular endothelial growth factor (VEGF) by cancer cells. As MPE indicates an incurable disease with limited palliative treatment options and poor outcome, there is an urgent need for new and efficient treatment options.
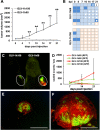
Methods: In this study, we used subcutaneously generated PC14PE6 lung adenocarcinoma xenografts in athymic mice that developed subcutaneous malignant effusions (ME) which mimic pleural effusions of the orthotopic model. Using this approach monitoring of therapeutic intervention was facilitated by direct observation of subcutaneous ME formation without the need of sacrificing mice or special imaging equipment as in case of MPE. Further, we tested oncolytic virotherapy using Vaccinia virus as a novel treatment modality against ME in this subcutaneous PC14PE6 xenograft model of advanced lung adenocarcinoma.
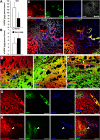
Results: We demonstrated significant therapeutic efficacy of Vaccinia virus treatment of both advanced lung adenocarcinoma and tumor-associated ME. We attribute the efficacy to the virus-mediated reduction of tumor cell-derived VEGF levels in tumors, decreased invasion of tumor cells into the peritumoral tissue, and to viral infection of the blood vessel-invading tumor cells. Moreover, we showed that the use of oncolytic Vaccinia virus encoding for a single-chain antibody (scAb) against VEGF (GLAF-1) significantly enhanced mono-therapy of oncolytic treatment.
Conclusions: Here, we demonstrate for the first time that oncolytic virotherapy using tumor-specific Vaccinia virus represents a novel and promising treatment modality for therapy of ME associated with advanced lung cancer.
Figures
Similar articles
-
Evaluation of a new recombinant oncolytic vaccinia virus strain GLV-5b451 for feline mammary carcinoma therapy.PLoS One. 2014 Aug 5;9(8):e104337. doi: 10.1371/journal.pone.0104337. eCollection 2014. PLoS One. 2014. PMID: 25093734 Free PMC article.
-
Virotherapy of canine tumors with oncolytic vaccinia virus GLV-1h109 expressing an anti-VEGF single-chain antibody.PLoS One. 2012;7(10):e47472. doi: 10.1371/journal.pone.0047472. Epub 2012 Oct 16. PLoS One. 2012. PMID: 23091626 Free PMC article.
-
Combination treatment with oncolytic Vaccinia virus and cyclophosphamide results in synergistic antitumor effects in human lung adenocarcinoma bearing mice.J Transl Med. 2014 Jul 17;12:197. doi: 10.1186/1479-5876-12-197. J Transl Med. 2014. PMID: 25030093 Free PMC article.
-
Oncolytic viruses: a promising therapy for malignant pleural effusion and solid tumors.Front Immunol. 2025 Apr 25;16:1570698. doi: 10.3389/fimmu.2025.1570698. eCollection 2025. Front Immunol. 2025. PMID: 40352942 Free PMC article. Review.
-
Fighting Fire With Fire: Oncolytic Virotherapy for Thoracic Malignancies.Ann Surg Oncol. 2021 May;28(5):2715-2727. doi: 10.1245/s10434-020-09477-4. Epub 2021 Feb 11. Ann Surg Oncol. 2021. PMID: 33575873 Free PMC article. Review.
Cited by
-
Dendritic cell factor 1 inhibits proliferation and migration and induces apoptosis of neuroblastoma cells by inhibiting the ERK signaling pathway.Oncol Rep. 2019 Jan;41(1):103-112. doi: 10.3892/or.2018.6796. Epub 2018 Oct 16. Oncol Rep. 2019. PMID: 30365123 Free PMC article.
-
Beyond cancer cells: Targeting the tumor microenvironment with gene therapy and armed oncolytic virus.Mol Ther. 2021 May 5;29(5):1668-1682. doi: 10.1016/j.ymthe.2021.04.015. Epub 2021 Apr 19. Mol Ther. 2021. PMID: 33845199 Free PMC article. Review.
-
Sirt3 Promoted DNA Damage Repair and Radioresistance Through ATM-Chk2 in Non-small Cell Lung Cancer Cells.J Cancer. 2021 Jul 13;12(18):5464-5472. doi: 10.7150/jca.53173. eCollection 2021. J Cancer. 2021. PMID: 34405009 Free PMC article.
-
Evaluation of a new recombinant oncolytic vaccinia virus strain GLV-5b451 for feline mammary carcinoma therapy.PLoS One. 2014 Aug 5;9(8):e104337. doi: 10.1371/journal.pone.0104337. eCollection 2014. PLoS One. 2014. PMID: 25093734 Free PMC article.
-
Viro-antibody therapy: engineering oncolytic viruses for genetic delivery of diverse antibody-based biotherapeutics.MAbs. 2021 Jan-Dec;13(1):1982447. doi: 10.1080/19420862.2021.1982447. MAbs. 2021. PMID: 34747345 Free PMC article. Review.
References
-
- Bennett R, Maskell N. Management of malignant pleural effusions. Curr Opin Pulm Med. 2005;11:296–300. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

