Ovarian follicle culture: advances and challenges for human and nonhuman primates
- PMID: 23635350
- PMCID: PMC3929501
- DOI: 10.1016/j.fertnstert.2013.03.043
Ovarian follicle culture: advances and challenges for human and nonhuman primates
Abstract
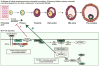
The removal and cryostorage of ovarian cortical biopsies is now offered as a fertility preservation option for young women. The only available option to restore fertility using this tissue is by transplantation, which may not be possible for all patients. The full potential of this tissue to restore fertility could be achieved by the development of in vitro systems that support oocyte development from the most immature stages to maturation. The techniques of in vitro growth (IVG) combined with in vitro maturation (IVM) are being developed with human tissue, but comparing different systems has been difficult because of the scarcity of tissue so nonhuman primates are being used as model systems. There are many challenges to developing a complete culture system that would support human oocyte development, and this review outlines the approaches being taken by several groups using tissue from women and nonhuman primate models to support each of the stages of oocyte development.
Copyright © 2013. Published by Elsevier Inc.
Figures



Similar articles
-
In vitro growth and maturation of primordial follicles and immature oocytes.Fertil Steril. 2021 May;115(5):1116-1125. doi: 10.1016/j.fertnstert.2021.03.004. Epub 2021 Apr 3. Fertil Steril. 2021. PMID: 33823993 Review.
-
In vitro oocyte maturation and preantral follicle culture from the luteal-phase baboon ovary produce mature oocytes.Biol Reprod. 2011 Apr;84(4):689-97. doi: 10.1095/biolreprod.110.088674. Epub 2010 Dec 1. Biol Reprod. 2011. PMID: 21123815 Free PMC article.
-
FERTILITY PRESERVATION: Progress and prospects for developing human immature oocytes in vitro.Reproduction. 2019 Nov;158(5):F45-F54. doi: 10.1530/REP-19-0077. Reproduction. 2019. PMID: 31557725 Review.
-
Matrix-free 3D culture supports human follicular development from the unilaminar to the antral stage in vitro yielding morphologically normal metaphase II oocytes.Hum Reprod. 2021 Apr 20;36(5):1326-1338. doi: 10.1093/humrep/deab003. Hum Reprod. 2021. PMID: 33681988 Free PMC article.
-
Encapsulated three-dimensional culture supports development of nonhuman primate secondary follicles.Biol Reprod. 2009 Sep;81(3):587-94. doi: 10.1095/biolreprod.108.074732. Epub 2009 May 27. Biol Reprod. 2009. PMID: 19474063 Free PMC article.
Cited by
-
Human Follicle in vitro Culture Including Activation, Growth, and Maturation: A Review of Research Progress.Front Endocrinol (Lausanne). 2020 Aug 11;11:548. doi: 10.3389/fendo.2020.00548. eCollection 2020. Front Endocrinol (Lausanne). 2020. PMID: 32849312 Free PMC article. Review.
-
Dynamic Characterization of the Biomechanical Behaviour of Bovine Ovarian Cortical Tissue and Its Short-Term Effect on Ovarian Tissue and Follicles.Materials (Basel). 2020 Aug 25;13(17):3759. doi: 10.3390/ma13173759. Materials (Basel). 2020. PMID: 32854374 Free PMC article.
-
Hypoxia Limits the Growth of Bovine Follicles in Vitro by Inhibiting Estrogen Receptor α.Animals (Basel). 2019 Aug 13;9(8):551. doi: 10.3390/ani9080551. Animals (Basel). 2019. PMID: 31412668 Free PMC article.
-
Recent advances: fertility preservation and fertility restoration options for males and females.Fac Rev. 2021 Jun 10;10:55. doi: 10.12703/r/10-55. eCollection 2021. Fac Rev. 2021. PMID: 34195694 Free PMC article. Review.
-
Prospects for fertility preservation: the ovarian organ function reconstruction techniques for oogenesis, growth and maturation in vitro.Front Physiol. 2023 May 12;14:1177443. doi: 10.3389/fphys.2023.1177443. eCollection 2023. Front Physiol. 2023. PMID: 37250136 Free PMC article. Review.
References
-
- Donnez J, Silber S, Andersen CY, Demeestere I, Piver P, Meirow D, Pellicer A, Dolmans MM. Children born after autotransplantation of cryopreserved ovarian tissue. A review of 13 live births. Ann Med. 2011;43:437–50. - PubMed
-
- Andersen CY, Kristensen SG, Greve T, Schmidt KT. Cryopreservation of ovarian tissue for fertility preservation in young female oncological patients. Future Oncol. 2012:8595–608. - PubMed
-
- Silber SJ, Barbey N. Scientific molecular basis for treatment of reproductive failure in the human: an insight into the future. Biochim Biophys Acta. 2012;1822:1981–96. - PubMed
-
- Eppig JJ, O’Brien MJ. Development in vitro of mouse oocytes from primordial follicles. Biol Reprod. 1996;54:197–207. - PubMed
Publication types
MeSH terms
Grants and funding
- RL1 HD058294/HD/NICHD NIH HHS/United States
- R01-HD058294/HD/NICHD NIH HHS/United States
- U54-HD18185/HD/NICHD NIH HHS/United States
- PL1 EB008542/EB/NIBIB NIH HHS/United States
- G0901839/MRC_/Medical Research Council/United Kingdom
- UL1 RR024926/RR/NCRR NIH HHS/United States
- HD058295/HD/NICHD NIH HHS/United States
- R01-HD058293/HD/NICHD NIH HHS/United States
- U54 HD018185/HD/NICHD NIH HHS/United States
- PL1-EB008542/EB/NIBIB NIH HHS/United States
- P51 OD011092/OD/NIH HHS/United States
- G0901839/1/MRC_/Medical Research Council/United Kingdom
- RL1 HD058293/HD/NICHD NIH HHS/United States
- RL1 HD058295/HD/NICHD NIH HHS/United States
- 8P51OD011092/OD/NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

