EMT impairs breast carcinoma cell susceptibility to CTL-mediated lysis through autophagy induction
- PMID: 23635487
- PMCID: PMC3722321
- DOI: 10.4161/auto.24728
EMT impairs breast carcinoma cell susceptibility to CTL-mediated lysis through autophagy induction
Abstract
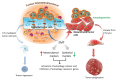
Epithelial to mesenchymal transition (EMT) has become one of the most exciting fields in cancer biology. While its role in cancer cell invasion, metastasis and drug resistance is well established, the molecular basis of EMT-induced immune escape remains unknown. We recently reported that EMT coordinately regulates target cell recognition and sensitivity to specific lysis. In addition to the well-characterized role for EMT in tumor phenotypic change including a tumor-initiating cell phenotype, we provided evidence indicating that EMT-induced tumor cell resistance to cytotoxic T-lymphocytes (CTLs) also correlates with autophagy induction. Silencing of BECN1 in target cells that have gone through the EMT restored CTL susceptibility to CTL-induced lysis. Although EMT may represent a critical target for the development of novel immunotherapy approaches, a more detailed understanding of the inter-relationship between EMT and autophagy and their reciprocal regulation will be a key determinant in the rational approach to future tumor immunotherapy design.
Keywords: autophagy; breast cancer; cytotoxic T-lymphocytes; epithelial mesenchymal transition.
Figures
Comment on
- Akalay I, Janji B, Hasmim M, Noman MZ, André F, De Cremoux P, et al. Epithelial-to-mesenchymal transition and autophagy induction in breast carcinoma promote escape from T cell-mediated lysis. Cancer Res. 2013;73:2418–27. doi: 10.1158/0008-5472.CAN-12-2432. doi: 10.1158/0008-5472.CAN-12-2432
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical