Kinetics and isotherm of fibronectin adsorption to three-dimensional porous chitosan scaffolds explored by ¹²⁵I-radiolabelling
- PMID: 23635535
- PMCID: PMC3749804
- DOI: 10.4161/biom.24791
Kinetics and isotherm of fibronectin adsorption to three-dimensional porous chitosan scaffolds explored by ¹²⁵I-radiolabelling
Abstract
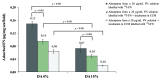
In this study, (125)I-radiolabelling was explored to follow the kinetics and isotherm of fibronectin (FN) adsorption to porous polymeric scaffolds, as well as to assess the elution and exchangeability of pre-adsorbed FN following incubation in serum-containing culture medium. Chitosan (CH) porous scaffolds with two different degrees of acetylation (DA 4% and 15%) were incubated in FN solutions with concentrations ranging from 5 to 50 µg/mL. The kinetic and isotherm of FN adsorption to CH were successfully followed using (125)I-FN as a tracer molecule. While on DA 4% the levels of adsorbed FN increased linearly with FN solution concentration, on DA 15% a saturation plateau was attained, and FN adsorbed amounts were significantly lower. These findings were supported by immunofluorescent studies that revealed, for the same FN solution concentration, higher levels of exposed cell-binding domains on DA 4% as compared with DA 15%. Following incubation in serum containing medium, DA 4% also revealed higher ability to exchange pre-adsorbed FN by new FN molecules from serum than DA 15%. In accordance, when assessing the efficacy of passively adsorbed FN to promote endothelial cell (EC) adhesion to CH, ECs were found to adhere at higher levels to DA 4% as compared with DA 15%, 5 µg/mL of FN being already efficient in promoting cell adhesion and cytoskeletal organization on CH with DA 4%. Taken together the results show that protein radiolabelling can be used as an effective tool to study protein adsorption to porous polymeric scaffolds, both from single and complex protein solutions.
Keywords: 3-D scaffolds; fibronectin; fluorescence; protein adsorption; protein conformation.
Figures





References
-
- Ratner BD, Hoffman AS, Schoen FJ, Lemons JE, eds. Biomaterials science: an introduction to materials in medicine. San Diego: Elsevier Academic Press, 2004.
-
- Chinn JA, Slack SM. Biomaterials: Protein Surface Interactions. In: Bronzino JD, ed. The Biomedical Engineering Handbook. Boca Raton: CRC Press LLC, 2000:1597-608.
-
- Dee KC, Puleo DA, Bizios R. Protein-Surface Interactions. In: Dee KC, Puleo DA, Bizios R, eds. An Introduction To Tissue-Biomaterial Interactions. New York, USA: John Wiley & Sons, Inc, 2003:37-52.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous
